



Metals and their compounds have been used for medical applications throughout history and it is interesting here to take a brief look at some of the milestones. Some of the earliest examples date from as far back as 3000 BC, when the ancient Egyptians were using copper sulfate to sterilise the water used in their tonics. There are also reports of gold-based medicines being used in China and Arabia around 2500 BC. Gold has also been used in dentistry; excavated graves in Italy dating from 1000 to 400 BC were found to have skulls containing gold bridges. Zinc was also reportedly used to promote the healing of wounds by the Romans.
Mercury has been in use in medicine for around 2000 years with a rather chequered history; mercury(I) chloride was traditionally used in the 16th century as a diuretic and laxative throughout Europe, and was also used to treat syphilis, often effectively poisoning the patient. By the 19th century HgCl was incorporated in a tonic known as ‘blue mass’ and prescribed for many ailments including such diverse conditions as constipation, toothache and depression. The use of mercury compounds is now largely forbidden because of their poisonous properties, but they are still to be found in traditional therapies such as Chinese, Tibetan and Ayurvedic medicines. The semimetal or metalloid, arsenic, well known as a poison, was also used for medicinal purposes; it was prescribed for a range of ailments, such as rheumatism, malaria, tuberculosis and diabetes. The medicinal use of arsenic sulfides was described by the ancient physician Hippocrates; however, it was in the 18th century that it became popular, prescribed as ‘Dr Fowler’s solution’, a mixture of potassium arsenite and lavender water. This was typically taken with wine as a general tonic and aphrodisiac.
It was not until the 20th century, however, that metal complexes began to be screened more systematically for their medicinal properties and in 1909 arsphenamine (salvarsan) became the first modern chemotherapeutic agent for treatment of syphilis. The structure of this drug has only recently been determined (Lloyd et al., 2005), almost 100 years after it was first used (Figure 9.1). This drug was later superseded by antibiotics. A lack of vitamin B12, a cobalt-containing protein (see Chapter 7), was found to be the cause of pernicious anaemia. This protein was first isolated in 1948 and its structure later confirmed by Dorothy Hodgkin using protein X-ray crystallography; for this and for the structure of penicillin, she was awarded the Nobel Prize in 1964.
Probably the most famous metal-containing complex to be found in the 20th century, however, is that of the platinum-containing anticancer drug, cisplatin. Its anticancer activity was discovered serendipitously by Barnett Rosenberg in the 1960s and it went on to revolutionise the treatment of some cancers, notably that of testicular cancer. Intensive research in this area has since spawned the development of other metal complexes for cancer therapy. It was the 20th century that also saw a growth in the use of radioactive metals to treat certain types of cancers, such as bone cancer.
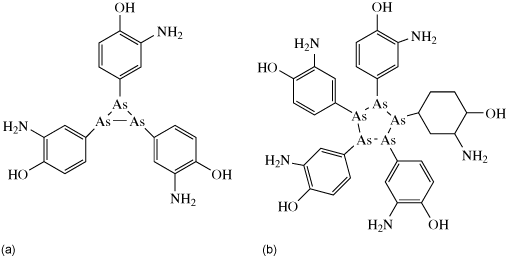
Figure 9.1 Two phenyl arsenic compounds found in the crystal structure of arsphenamine.
Activity 9.1 gives you the opportunity to read an overview of this subject in the opening chapter of Medicinal Applications of Coordination Chemistry, by C.J. Jones and J.R. Thornback, (Jones and Thornback, 2007), one of the Royal Society of Chemistry eBooks that we will be using throughout this chapter.
Read Section 1.1 of the opening chapter of Medicinal Applications of Coordination Chemistry (Jones and Thornback, 2007), pages 1–3. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.1
Other than as therapeutic agents what other major use do metals find in medicine?
Metals are also used for diagnostic applications, in particular in imaging.
End of answer
As you have seen in this brief introduction the number of metal-containing compounds that have found use as therapeutic and diagnostic agents for medical applications is ever expanding, and it will not be possible in a single chapter to discuss the chemistry of all of these in detail. What we will aim to do here is to highlight examples of some of the different areas in which metal complexes are important and to demonstrate the particular chemical and physical properties that make them useful. The text in this online chapter will be supplemented by video material and also sections of texts from books in the Royal Society of Chemistry eBook collection which support this topic area.
First, we will look at the use of metals as pharmaceuticals in general. We will then consider metal homeostasis, looking in particular at the nature of those diseases which arise when metals are either deficient or present in excess and will see what treatments are available. Next, we will consider the use of metals in imaging. Magnetic resonance imaging (MRI) is widely used for the diagnosis of many ailments and it has been found that certain gadolinium complexes can be used to enhance the contrast of the image. We will also take a detailed look at the use of radioactive nuclei to monitor the functioning of particular organs in the body. Finally, we shall consider metals as therapeutic agents, in particular those used for the treatment of cancer. This detailed study will consider both the mechanism of operation of cisplatin and other metal anticancer drugs used in chemotherapy, and also the use of radioactive nuclei in radiotherapy.
Before we start to explore some of the different examples of the use of metals for medical applications, we should consider the general factors that arise in the design of metal based pharmaceuticals. Activity 9.2 gives you the opportunity to read an overview of this subject, again from the opening chapter of Medicinal Applications of Coordination Chemistry.
Read Sections 1.3 and 1.4 from Chapter 1 of Medicinal Applications of Coordination Chemistry (Jones and Thornback, 2007), pages 6–14, then answer the following questions. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.2
What factors determine the pharmokinetic behaviour of a drug?
The pharmokinetic behaviour of a drug depends on: the rates of absorption, distribution, metabolism and elimination.
End of answer
Summarise these processes for the example of a drug injected intravenously into the body, highlighting key issues that might arise at each point.
When a drug is injected intravenously into the body, the drug is injected directly into the bloodstream. Here, the solubility of the drug can be an issue, along with competitive complexation with proteins in the bloodstream. In some examples this complexation might be desirable to aid distribution of the drug to where it is required, in others it can be detrimental. The drug will need to be sufficiently stable so that it can have the desired effect before being broken down by metabolic processes and excreted, but not so stable as to remain in the body to become toxic. In many examples, the drug may actually need to be metabolised to produce the active form of the drug.
End of answer
What is meant by the term prodrug?
A prodrug is an example of a drug where the form administered is metabolised in the body to produce the active form.
End of answer
In the next two sections we will return to the role of metals in the body and in particular the problems that can arise from too little or too much of a metal and how this can be treated.
We have seen throughout this book just how vital metals are for many of the key biological processes in organisms. It is therefore not surprising that a deficiency (or excess) of certain metals can severely compromise an organism, as we addressed briefly in Chapter 1. Perhaps the most common example of a metal deficiency is that of iron, causing anaemia, which leads to fatigue and an increased chance of infection. Table 9.1 lists the recommended daily intakes for some of the essential metals, together with some of the effects of deficiencies in these metals (reproduced from Table 1.1).
Table 9.1 UK Food Standards Agency reference nutrient intake (RNI) for adults (and the US Department of Agriculture dietary reference intakes (DRI)) and the effect of deficiency for selected essential metals.
| Metal | NRI (DRI)/mg | Effect of deficiency | |
|---|---|---|---|
| Male | Female | ||
| potassium | 3500 (4700) | 3500 (4700) | * |
| sodium | 1600 (1500) | 1600 (1500) | * |
| calcium | 700 (1000) | 700 (1000) | retarded skeletal growth |
| magnesium | 300 (400) | 270 (310) | muscle cramps |
| iron | 8.7 (8) | 14.8 (18) | anaemia, immune system disorders |
| zinc | 5.5–9.5 (11) | 4–7 (8) | skin damage, stunted growth |
| copper | 1.2 (0.9) | 1.2 (0.9) | liver disorder, artery weakness |
| chromium | 0.025 (0.035) | 0.025 (0.025) | diabetes symptoms |
| cobalt** | 0.0015 (0.0024) | 0.0015 (0.0024) | pernicious anaemia |
* deficiency is rare and acute cases only tend to occur with severe dehydration.
** Supplied as vitamin B12.
In humans, deficiencies in metals from our diet can be offset by taking mineral supplements. You should read the article from the RSC eBook, Trace Elements Medicine and Chelation Therapy, by D.R. Williams and D.M. Taylor (Williams and Taylor, 1995) in the following Activity which looks at supplements in some detail.
Read Chapter 5 of Trace Elements Medicine and Chelation Therapy (Williams and Taylor, 1995), pages 50–56, and answer the questions below. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.3
The majority of the iron supplements discussed in the article are provided as the Fe(II) salt, why might this be?
Fe(II) is generally more soluble than the corresponding Fe(III) salt.
End of answer
Why is cobalt required as cobalamin, a form of vitamin B12?
Cobalamin can not be synthesised by the body and so must be taken in our diet in this form.
End of answer
Figure 9.2 shows the form of the supplements in one brand of multivitamins. Many of the salts are provided as the sulfate; why might this be?
In general, the sulfates tend to be soluble.
End of answer
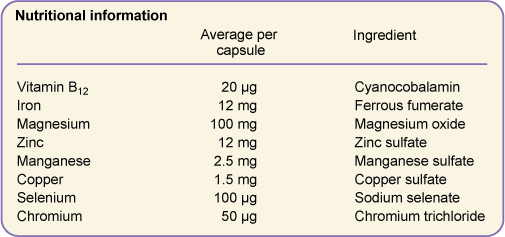
Figure 9.2 Ingredients in a commercial brand of multivitamins.
More sophisticated approaches are sometimes required for the treatment of specific diseases that arise due to the failure of the body to manage levels of essential metals. One such example is Menkes disease, a rare but fatal genetic disorder affecting about 1 in 250 000 baby boys. Problems in intracellular Cu transport (in particular, uptake by the intestines via Cu–ATPase) cause a deficiency in copper leading to retarded growth and mental retardation in sufferers of this disease. Early treatment is essential; however the disease is not usually evident until the infant is around 6–8 weeks old when symptoms first become apparent. A characteristic symptom of the condition is colourless, or steel coloured, kinky hair, leading to the disease sometimes being called ‘kinky hair’ syndrome. Left untreated, children will usually die before the age of three.
Why are copper supplements ineffective in treatment of deficiency for sufferers of this disease?
As the copper uptake mechanism is not functioning properly in sufferers of Menkes disease, there would be little point taking traditional supplements, such as copper(II) sulfate.
End of answer
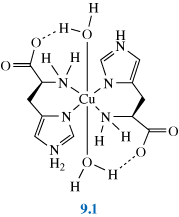
The condition can be treated by subcutaneous injections (under the skin) of copper histidine complexes, such as 9.1. (This form of treatment was developed after it was discovered that copper histidine complexes bind to albumin in human blood serum providing exchangeable copper.) Although early treatment can prevent some of the neurodegenerative problems associated with the disease, it does not eliminate tissue disorders, also a consequence of this disease.
In this section we will consider the effect of too high a level of metal; metal overload. Excess levels of metals in the body can cause toxic effects, and can be fatal. You may well be aware of the notorious use of some metals throughout history as poisons. The effects of high levels of some metals and guidance levels for safe consumption (without ill effect) are given in Table 9.2. Considering the example of iron yet again, high levels of iron can lead to cirrhosis of the liver, damage to the heart and ultimately death. Indeed iron poisoning (from accidental overdose of iron supplements) is one of the leading causes of death in young children from poisons in the US, with toxic levels at just 100–200 mg of iron per kg body weight.
Table 9.2 Safe upper level (or guidance level) and effect of excess for selected metals.
| Metal | Safe upper level per day/mg (adult) | Problem |
|---|---|---|
| potassium | * (3700) | stomach pain, nausea, diarrhoea |
| sodium | * (2300) | increased blood pressure (hypertension) increasing risk of heart attack or strokes |
| calcium | * (1500) | stomach pain, diarrhoea, kidney stones, milk alkali syndrome |
| magnesium | * (400) | diarrhoea |
| iron | * (17) | constipation, stomach pain, cirrhosis of liver, impaired heart function and failure |
| zinc | 25 | reduces absorption of copper and iron, leading to anaemia and weakening of bones |
| copper | 10 | stomach pain, damage to the liver and kidneys. |
* Insufficient data to establish a safe upper level – value given is a guidance value for supplemental doses producing no adverse reaction.
Before we look at metal overload in detail, you should recall that we met some of the methods that cells use to avoid toxic levels of metals in Chapter 5, Section 5.5.
What main methods are employed by cells to avoid metal overload?
Cells can prevent accumulation of toxic levels of metals by excluding the metals from the cell, extrusion by pumping metal ions out of the cytoplasm, and detoxification by rendering the metal harmless by chelation or metabolising it into a less toxic form.
End of answer
In Chapter 1 we discussed thalassemia, a disease which causes an abnormality in the globin portion of haemoglobin, causing anaemia. Treatment for thalassemia typically involves blood transfusions, which can cause toxic levels of iron to accumulate in the body.
What method would you suggest could be used to remove excess iron from the body?
One method would be chelation of the iron by a suitable ligand.
End of answer
The most common form of treatment for thalassemia sufferers is the intravenous application of the chelator Desferal® (desferrioxamine B), 9.2. Desferrioxamine B is a siderophore, originally obtained from a metabolite of the bacterium, Streptomyces Pelosus.
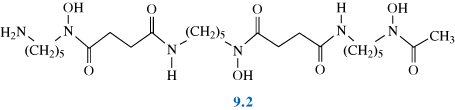
What properties of siderophores, such as in the example here, enable them to be such effective ligands for iron and other metals?
Desferrioxamine B contains hydroxamate groups, and like siderophores in general, is a hexadentate ligand, forming an octahedral arrangement around the metal ion. Fe(III)–siderophore complexes typically have high stability constants (K = 30.6 for desferrioxamine B). The corresponding Fe(II)–siderophore complexes tend to be significantly less stable.
End of answer
What other (non-siderophore) ligand have we met previously that has a high stability constant for the chelation of Fe(III)?
edta4–.
End of answer
This ligand is not generally used to remove Fe. So, what influences the use of a particular chelating ligand for the treatment of metal overload?
Activity 9.4 looks at the use of chelating agents in general and considers what influences the choice of ligand as a chelating agent. It also discusses how chelation therapy is used to treat diseases such as thalassemia and also Wilson’s disease, another disorder, which, like Menkes disease, affects Cu transport. In Wilson’s disease, toxic levels of copper accumulate in the cytosol of liver cells, leading to release of copper into the bloodstream and eventually accumulation of copper in other organs, such as the brain.
Read Sections 4.2 of Medical Applications of Coordination Chemistry (Jones and Thornback, 2007), pages 202–218, and then answer the following questions. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.4
(The structures of two of the ligands discussed in the extract are not included in the extract itself, but are shown in Figure 3.1a (edta4–) and Structure 9.4 (DTPAH5).) Please note that the H in the various abbreviations used in the extract refers to the number of protons in the ligands (the higher the number in a series the less deprotonated the ligand).
Why is edta4– not a suitable ligand for use in chelation therapy for the removal of excess iron?
Edta4– is not selective for iron and will also form stable complexes with other essential metals, for example Ca and Zn.
End of answer
Summarise the key criteria that are important in the design of new chelators for Fe?
The chelator should have a high affinity for iron, and should be selective for iron, compared to other essential metals such as Zn, Ca, Mg and Cu. It should also have a low toxicity and should be rapidly excreted on forming the Fe-complex. Finally, it should have a low cost and preferably should be administered orally rather than by injection.
End of answer
Many of the important advances in medicine in recent decades have arisen from our progress in understanding the structure and workings of the human body. Our ability to visualise internal structures has led to increased precision in both diagnosis and treatment. We can pinpoint the causes of disease and monitor the progress of any intervention. Medical imaging therefore has become one of the most important weapons in our fight against disease.
Diagnosis of illness is aided by the ability to obtain detailed information about the structure of a particular bone or organ to see if it is abnormal, damaged or malfunctioning in some way. For example, a growth may prevent the passage of food and waste through the gut or fatty deposits may cause problems with blood circulation. To obtain this information, the abnormal or diseased tissue has to stand out from those around it – its properties have to be sufficiently different from the properties of normal tissue or surrounding matter, to be distinguished by the techniques chosen for investigation. We are probably most familiar with X-ray images, which are commonly used to look at broken bones, but other methods include the use of ?-rays, ultrasound waves and magnetic resonance (MRI).
There are two main types of imaging which we will consider in turn: anatomical imaging where the structures of the body are examined by exploiting differences in the physical or chemical properties of the materials in the body, for example between bones and soft tissue, or normal breast tissue and breast tumour, and functional imaging where, for example, a substance can be injected into the body and its distribution tracked and monitored to assess the functioning of a particular organ or system. We will look at the first of these, anatomical imaging, in the next two sections.
X-rays are routinely used in diagnosis, for example in examining broken bones. X-rays are part of the electromagnetic spectrum and have the potential to interact with matter, either atoms or molecules. The nature of the interaction depends on the energy of the radiation concerned. As X-rays pass through matter, they may go straight through unimpeded, they may be absorbed or they may be scattered and carry on in a slightly different direction. Absorption of X-rays can excite the core electrons in an atom, giving them sufficient energy to be ejected from the atom; X-rays are often referred to as an ionising radiation. The greater the atomic number of an element, the more strongly it absorbs X-rays.
X-rays can also be scattered by the electrons in atoms, and this will also increase with increasing atomic number. (Which is the dominant process, will depend on the energy range used for imaging.) In addition, the more dense the matter, the more opportunities there are for interactions leading to absorption or scattering. The overall attenuation, loss of intensity of the X-ray beam, thus depends on atomic number, density and, of course, the thickness of the sample. To achieve a good image there must be a difference in the attenuation between the different tissues. Most body tissue is made from water and carbon-based polymers containing low atomic number atoms, mainly carbon, nitrogen, oxygen and hydrogen. Bones absorbs X-rays more strongly. (Figure 9.3a); it not only contains elements with higher atomic number than the surrounding soft tissue, such as calcium and phosphorus, but also is denser – a solid with closely bonded atoms. Some types of tissue, for example breast tumours which are denser than the surrounding fatty breast tissue, can also be differentiated by X-rays (Figure 9.3b).
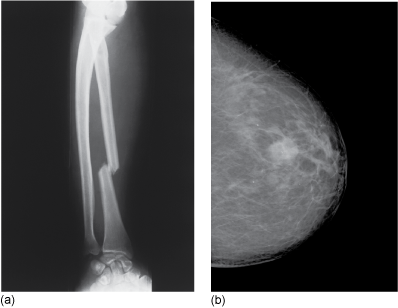
Figure 9.3 X-ray of (a) a broken bone and (b) a breast tumour.
Source: (a) James Stevenson/Science Photo Library. (b) Royal Berkshire NHS Foundation Trust.
A conventional X-ray of a bone fracture is a two-dimensional (2D) image, taken from the front of the patient by a single camera and is sometimes known as a planar X-ray. A computed tomography (CT) scan produces many 2D images of sections throughout the body using detectors arranged in a circular field, which can then be computer processed to give a 3D reconstruction of the body. With carefully controlled conditions, even changes in soft tissues indicating tumours can be picked up and located by this method. The resolution can be as good as 1 mm or less.
The following video clip shows a CT scan being performed in a hospital for a patient with a suspected injury to his spine.
One way of improving the differentiation between tissues when using X-rays is to use a contrast agent. This is a substance, in this instance, that preferentially absorbs X-rays and hence shows up more clearly the organs into which it is injected or introduced. (Another type of contrast agent is used in magnetic resonance imaging as you will see in Section 9.6.2.)
Can you suggest one property of an X-ray contrast agent that would influence its absorbance?
The contrast agent should have a high atomic number, since this will lead to greater absorbance.
End of answer
There are a variety of these agents available, the oldest of which is barium. Barium has a high atomic number and absorbs X-rays extremely well. It also possesses an extremely insoluble compound, barium sulfate, BaSO4. A ‘barium meal’ is given to patients to swallow in the form of a milky-looking drink, and its progress through the intestinal tract is followed with X-rays. This is typically used to visualise the structures of the upper gastro-intestinal (GI) tract. For the lower parts of the intestines, including the bowel, a barium enema is given instead as this saves time.
Why is it desirable for this contrast agent to be insoluble?
The very low solubility product of BaSO4 (1.1 × 10–10) means that this is not absorbed in the body but is simply excreted with no danger – soluble salts of barium are in fact poisonous.
End of answer
Such things as ulcers in the stomach wall and abnormal growths can be picked up using a barium meal. Figure 9.4 shows an X-ray of the large intestine for a patient using this method.

Figure 9.4 Image of a barium meal passing through the bowel. You should note that the contrast of the image has been reversed to see the intestines better and hence allow the medical practitioner to make a diagnosis.
Source: University Hospitals Coventry and Warwickshire NHS Trust.
Contrast agents are also used to enhance the image in organs such as the kidneys, liver and bladder as well as in bronchography. More recent developments in this area have seen the introduction of a range of tri-iodinated benzenoid compounds, for example Gastrografin®, 9.3, which are capable of more targeted action and so can further improve the selectivity of the X-ray technique. Polymetallic tungsten complexes such as K3[PW12O40] are also being investigated for some applications.
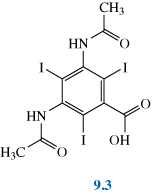
Why do you suppose such compounds are being employed/investigated as contrast agents?
Iodine and tungsten are heavy elements and so compounds containing a number of these atoms will absorb X-rays well.
End of answer
When having an X-ray taken, you are asked not to use talcum powder (a magnesium silicate) or antiperspirant (typically containing aluminium and often zirconium) on the day of the appointment. Why might this be so?
Magnesium, silicon, aluminium and zirconium are heavier elements than those contained in body tissue and so show up as spots on the film where they absorb the X-rays.
End of answer
The following short movie clip shows a CT scan of the head using a tri-iodated contrast agent. In this example the contrast agent has been preferentially taken up in the blood.
Magnetic resonance imaging, (MRI), is a non-invasive investigative technique, which produces a 3D image of the body, from a series of 2D images, by measuring the nuclear magnetic resonance (NMR) signals from mobile protons, mostly in the water present in the body but also the protons present in fats and proteins.
Read Section 3.2.1.1, pages 103–106 in the RSC eBook Medical Applications of Coordination Chemistry (Jones and Thornback, 2007). This gives a simplified account of the way MRI works. Then watch the following video to see MRI being used for diagnosis in a hospital. In this video the patient has a suspected head fracture (evident using X-ray) and MRI is being used to see if there is any damage to the brain. You should then answer the questions below. (A definition of the planes referred to in this video clip is given in Box 9.1.) Extract for Activity 9.5
Summarise the three key stages in the MRI process.
In summary, the MRI process has three stages:
End of answer
As we have seen above when a magnetic field is applied, protons try to align with or against the magnetic field. Because there are slightly more protons aligned parallel to the field (i.e. in the ground state), the tissue has an overall net magnetic moment, usually known as the net magnetisation vector in the same direction as the applied magnetic field. It is conventionally given the symbol Mz, where the subscript denotes the direction of magnetisation, in this case parallel to the external magnetic field.
When a radiofrequency pulse is applied it excites some of the protons into an excited state. In a classical picture, the effect of this interaction is that the net magnetisation vector rotates away from its original direction. The angle through which this rotates is defined by the duration of the radiofrequency (rf) pulse. Hence the pulse duration is described by its effect on the net magnetization vector, e.g. a 90° pulse rotates the magnetisation into the xy plane, and a 180° pulse rotates the magnetisation into the -z direction. In MRI experiments a 90° pulse is used.
Typically the radiofrequency is in the range 40–300 MHz, and the pulses have a duration of microseconds (2–10 µs). Unless you have a particular medical condition that requires a heart pacemaker, for example, neither the field nor the high-frequency radiation, in general, is harmful.
Although the basis of the MRI technique is 1H NMR, it is important to realise that it does not involve recording an NMR spectrum in order to analyse which molecules are present. A complete 1H NMR spectrum of the human body would show a large number of signals from protons in different proteins, DNA, etc. and from different parts of the body and would be impossible to interpret. At very low resolution, the proton spectrum of human tissue shows two broad peaks corresponding to water and fat.
Consider now the image shown in Figure 9.5. MRI is a particularly important technique, as shown in the video, for imaging the brain. Figure 9.5 shows a typical slice through the sagittal plane (Box 9.1), which clearly shows the skin, grey and white matter, cerebrospinal fluid and other components of the brain. The smallest detail in this image is a millimetre or less and images such as this are used to provide vital information as a means of diagnosis or to predict any surgical intervention.
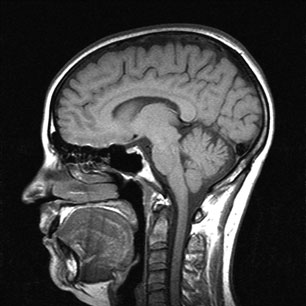
Figure 9.5 A typical slice through the brain in the sagittal plane.
Source: University Hospitals Coventry and Warwickshire NHS Trusts.
There are three directions in which slices through the brain (or the body in general) can be obtained in imaging as illustrated in Figure 9.6; axial, sagittal and coronal.
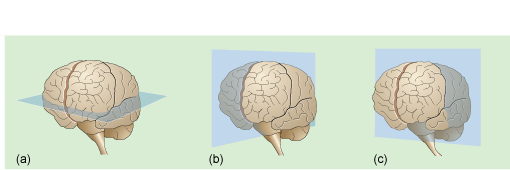
Figure 9.6 The three section planes: (a) axial ; (b) sagittal; (c) coronal through the brain.
So, how are different components, such as the grey and white matter, and cerebrospinal fluid in the brain identified using MRI? And how is the spatial localisation achieved, providing information about where in the brain the different components are located?
In terms of spatial localisation, a magnetic field gradient is applied across the body in three orthogonal directions. The external magnetic field experienced by a proton depends on where in the body that proton is situated. When a proton relaxes back to the ground state, the energy emitted will depend on the position of that proton in the magnetic field gradient and hence on its location in the body. A complicated sequence of pulses and gradient fields enables the signal to be localised. This allows a three-dimensional image of the distribution of protons to be created.
Both fat and water signals are detected at each position, but the magnetic field gradient is such that the range of frequencies required to excite protons is distinct for each volume element or voxel (a 3D pixel). To obtain an MRI image, the intensity of the NMR emission signal is recorded for each voxel, that is, as a function of position.
What does the signal intensity depend on?
It depends largely on the relaxation characteristics of the protons (and to a lesser extent the proton density).
End of answer
The relaxation characteristics will vary for the different tissues in the body. For example, muscle has a higher water content than fat. Similarly in the example of the brain shown in Figure 9.5 above, cerebrospinal fluid has a different water content to grey matter. It is the relaxation characteristics that are particularly important in determining the contrast of the image and we will consider the effect of relaxation in the next section.
The mechanisms whereby nuclear spins return to the ground state after they have been excited are known as relaxation, and there are two such mechanisms. The longitudinal relaxation time, or spin–lattice relaxation time, T1, is the recovery of the magnetisation to its thermal equilibrium along the direction of the main applied magnetic field (i.e. along the z-direction, Mz.) and is associated with the transfer of energy between the protons in the excited state and their surroundings. At room temperature, because the energy difference between the nuclear spin states is so small, just under half of the protons will be in the excited state. After a pulse of radiation is applied, there are more protons in the excited state. The rate at which the protons relax to the equilibrium state is given by the longitudinal relaxation rate, 1/T1. The larger the value of T1, the slower the return to equilibrium.
This is illustrated in the following animation which allows you to explore for yourself the recovery of magnetisation, Mz, after a single pulse of radiofrequency. It shows how the signal from tissues with different T1 relaxation time varies and allows you to choose the time at which the difference between two tissues, and therefore the contrast, is maximised. You will see for instance, that Mz. for fat tissue recovers more quickly than Mz. for water and so tissues containing different proportions of fat and water can be differentiated as they will have different signal intensities (so T1 (water) > T1 (fat)).
This graphical animation shows how T1 contrast in MRI varies between different tissue types. Choose two different tissues from the drop down boxes on the right and note how the difference in Mz varies.
The transverse relaxation time, or spin–spin relaxation time, T2, relates to the transfer of energy between protons in the ground state and those in the excited state. In this case the recovery measured is the loss of the magnetisation in the xy plane, Mxy. Immediately after the application of a 90° radiofrequency pulse, the net magnetisation vector in the xy-plane will be at its largest. Because of local variations in the static magnetic field, and interactions with other spins, the magnetisation in the xy-plane slowly reduces until it returns to zero. This effect of this type of relaxation is illustrated in the animation below.
Choose two different tissues and note how the difference in Mxy varies. Use water as one of the tissues and note the large difference between it and other substances (so T2 (water) > T2 (fat)).
As you saw above the protons in different tissues have different relaxation times (approximate values are given in Table 9.3). This is because the protons in different types of tissues will have different degrees of freedom or mobility, which will directly affect how readily they can interact with other species in their surroundings. For small molecules in solution, as in a conventional NMR experiment, T1 and T2 are roughly equal, but in the body where molecules are moving less freely, T2 in general tends to be much shorter than T1 (see Table 9.3).
Table 9.3 Approximate relaxation times of water protons in brain tissues at 1 T compared with fat and water.
| Tissue | T1 (mean)/ms | T2 (mean)/ms |
|---|---|---|
| fat | 250 | 80 |
| white matter | 650 | 90 |
| grey matter | 800 | 100 |
| cerebrospinal fluid (CSF) | 2000 | 150 |
| water | 3000 | 2500 |
The intensities of the tissues seen in an image will depend on their relaxation characteristics and the way in which the MRI sequence is set up.
Contrast can be enhanced by designing the pulse sequence so that either T1 or T2 effects dominate the relative intensities measured for different tissues. Images are said to be either T1-weighted – that is to say the image contrast depends largely on the differing T1 values of the tissues – or T2-weighted. In T1-weighted images, tissues that have lower T1 values (particularly fat) appear bright. In T2-weighted images, tissues that have greater T2 values appear brighter, (see Figure 9.7) – water therefore appears as very bright
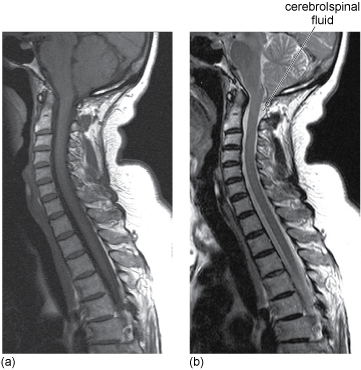
Figure 9.7 (a) T1-weighted image. (b) T2-weighted image of the spine for an elderly patient. Note the cerebrospinal fluid in particular is brighter in the T2-weighted image.
Source: University Hospitals Coventry and Warwickshire NHS Trust.
You may like to revisit the previous video clip now that you have met these terms, in particular the T1- and T2-weighted images.
It can be useful in diagnosis to be able to enhance the contrast even more. In the next section, we see how this can be done by injecting patients with artificial contrast agents.
MRI contrast agents, which of course must be non-toxic and excreted quickly from the body, change the relaxation times and thus the NMR signal intensity. Because they enter some types of tissue in preference to others and interact with the water in these tissues, they affect the relaxation properties of the protons in some tissues more than others. A substance that is preferentially taken up by one region of the body rather than another will thus enhance contrast in the image. As you will see, many of these contrast agents are paramagnetic metal complexes.
The ways in which paramagnetic metal complexes affect neighbouring water molecules is complicated. Paramagnetic metal ions have a magnetic moment by possessing one or more unpaired electrons. This magnetic moment will interact strongly with any magnetic fields to which it is exposed (indeed this is the basis of the method used to measure magnetic moments in the laboratory).
Of the high-spin metal ions, Fe3+, Cr3+, Mn2+ and Gd3+, which would you expect to have the largest number of unpaired electrons and hence magnetic moment? (Use the Periodic Table to work out their electronic configurations.)
The ions have 5, 3, 5 and 7 unpaired electrons, respectively, so Gd3+ would have the largest magnetic moment.
End of answer
As we are interested in the protons largely of the water molecules in the body, we need to consider the nature of the interactions between these and the metal ion. Water molecules can, of course, be bound directly to the metal, i.e. as a ligand, and in this position (which we describe as inner sphere coordination), they will be more influenced by the magnetic field of the metal ion. Other water molecules are bound further away from the metal ion, often hydrogen-bonded to one of the ligands (outer sphere). These will feel less of an influence from the magnetic field of the metal ion but are in a good position to interact with protons in the bulk of the surrounding tissues. For the contrast agent to affect the relaxation rates of protons in tissues, there must be a dynamic exchange of water molecules between the inner sphere, outer sphere and uncomplexed bulk water molecules. It is a short step then to realising that careful design of the ligands on a complex might enable us to target specifically certain types of organs or particular types of disease. That we are indeed able to do this is one of the triumphs of bioinorganic chemistry.
The following activity describes some of the key issues that affect the design of coordination complexes for use as contrast agents. You should read this now and then answer the following questions.
Read Sections 3.2.1.2. and 3.2.1.5–3.2.1.6, pages 106–109 and 113–115 of Medical Applications of Coordination Chemistry (Jones and Thornback, 2007), and then answer the following questions. This describes the use of paramagnetic MRI contrast agents and the requirements for a clinically useful contrast agent. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following links: Extract a for Activity 9.6 and Extract b for Activity 9.6
Contrast agents rely on a fast exchange of ligand water with the bulk. Describe the various types of linkages that water can make in such a complex.
The water can be present as both inner-sphere and outer-sphere ligands as well as in the surrounding aqueous environment in the body. Protons on the outer-sphere water may also effectively become inner sphere by hydrogen bonding to an inner sphere ligand such as water or a carboxylate. This is shown schematically in Figure 6 of the extract.
End of answer
How does such a dynamic exchange between an inner-sphere water ligand of the contrast agent and the surrounding aqueous environment affect the relaxation time of protons?
Dynamic exchange between the inner-sphere water ligand in the complex and the bulk aqueous environment for example in the affected tissue, will shorten the relaxation time, T1, of the protons in water in the affected tissue and thus increase the signal intensity.
End of answer
Briefly summarise the properties of a good MRI contrast agent.
A good MRI contrast agent will have a large magnetic moment, inner- and outer-sphere water ligands, a fast exchange rate between the water molecules, and be thermodynamically and kinetically stable. It should also, ideally, be non-toxic.
End of answer
The coordination complexes of which metal have been the most developed for use as a contrast agents? What has influenced the choice of this metal?
Gadolinium is the most widely used ion in MRI contrast agents as it has a high magnetic moment and long relaxation time. Its larger ionic radius (compared to other ions with high magnetic moments such as Mn2+) results in a larger coordination number. It is also less toxic than other ions.
End of answer
Gadolinium complexes have naturally been chosen as contrast agents because of their very high magnetic moments. Figure 9.8 shows an example of the marked improvement in the quality of the MRI data when a gadolinium contrast agent is used. The complex is delivered to the patient intravenously and is carried round in the blood plasma but does not enter the cells. Considering again the example of the brain, gadolinium complexes cannot cross the blood–brain barrier in a normal brain. However, in Figure 9.8 a tumour is present, leading to a breakdown of the blood–brain barrier and thus, allowing the contrast agent to cross into the brain.
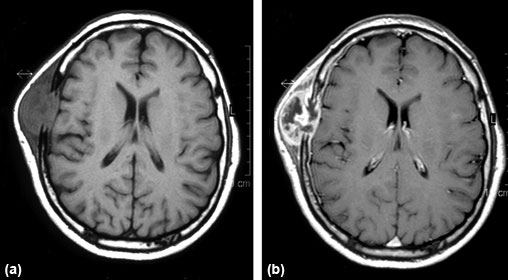
MRI images of a brain containing a tumour of the frontal bone (a) without and (b) with gadolinium contrast enhancement.
Source: El Saghir et al, 2005.
What factors influence the choice of ligand for a contrast agent?
The ligand should form a complex with a high stability constant. This can be influenced by the choice of polydentate ligands, ligand preorganisation and the choice of correct ligating atoms. The ligand also should be anionic as this will promote binding to the metal cation. In addition, an anionic complex will have greater interactions with outer sphere water.
End of answer
Gd3+ is a hard acid. Which coordinating atoms would be preferred for binding?
Gd3+ is likely to coordinate to hard bases such as O or N atoms.
End of answer
The first gadolinium complex to be used clinically, in 1988, was Magnevist®, a nine-coordinate complex of gadolinium(III) [Gd(DTPA)H2O]2–. The DTPA ligand is diethylenetriaminepentaacetate (ethanoate), shown as the protonated form in 9.4.

DTPA is an octadentate ligand, with coordination by all five ethanoate groups and the three nitrogens. A ninth coordination site on gadolinium is occupied by a water ligand.
What type of ligand is H2O in this structure?
It is an inner-sphere ligand as it is attached directly to the metal.
End of answer
DTPA also complexes well to other metals and is used as a chelating agent to remove heavy metals from people poisoned with plutonium, americium and other actinides.
Why is DTPA useful for the removal of actinides in particular?
With the large actinide ions, DTPA can act as an octadentate ligand. On formation of chelate complexes, the metal ions are less readily absorbed and can then be eliminated through the kidneys.
End of answer
This Gd(III) complex has since been followed by others as shown in Figure 9.9.
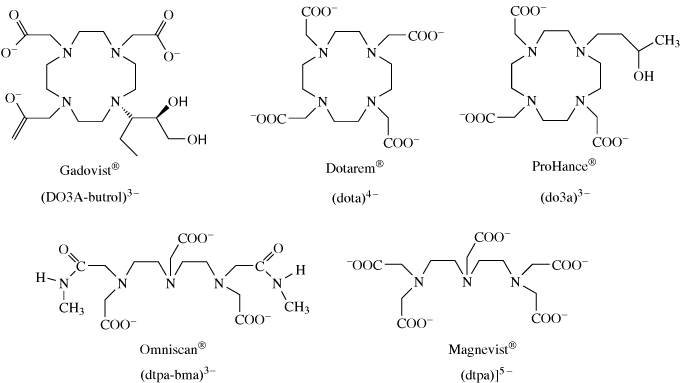
Figure 9.9 Ligands of Gd(III) complexes approved for clinical use as MRI contrast agents.
As we have seen there are certain criteria that are essential for a good contrast agent. Gadolinium is toxic to humans, so the complexes must be thermodynamically and kinetically stable and hence not break down releasing Gd3+ into the body.
Why do the ligands in Figure 9.9 fulfil this criterion?
The ligands are polydentate and in some cases macrocyclic. This will lead to greater stability compared to simple ligands such as NH3.
End of answer
As gadolinium is a very large atom, it can accommodate a large number of ligands; a single octadentate ligand such as DTPA ensures that there is not room for many water ligands to coordinate, as too many water ligands may make the complex too reactive.
The synthesis of such complexes, and their stability and reaction chemistry, is an important area of current research for inorganic chemists. The use of contrast agents has soared with about one-third of all MRI scans now using them. Other metals, with chemistries and magnetic properties similar to gadolinium, could also be promising contrast agents and complexes of Cr(III) and Mn(II) are currently being explored.
A further consideration is the rate at which the contrast agent rotates or tumbles in solution. A slow rotation rate can reduce relaxation times, although the relationship between rotation rate and relaxation rates is complex. One line of research is thus to employ large molecules such as proteins, as ligands, which tumble slowly in solution. Another possibility is to use antibodies as ligands to improve tissue targeting.
The following activity describes some of the key issues that affect the choice of ligand for coordination complexes for use as contrast agents. You should read this now and answer the following questions.
Read Section 3.2.1.7, pages 115–118 of Medical Applications of Coordination Chemistry, (Jones and Thornback, 2007), and then answer the questions below. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.7
Why is edta4– not a suitable ligand for Gd coordination in a 1:1 metal–ligand complex?
As a hexadentate ligand, edta4– alone will not saturate all the Gd3+ coordination shell in a 1:1 complex. This will leave room for other ligands, such as water, to coordinate.
End of answer
How does the effect of changing the substituents on a ligand influence the stability of the complex?
A substituent might destabilise a complex by inhibiting the metal–ligand binding. Alternatively, a substituent might increase the stability of a complex by preventing approach and coordination by competiting ligands. Additional stability may result if the substituent has donor atoms suitable for coordination and chelate formation. The rate of ligand dissociation, exemplified by the acid dissociation constant, Ka, can also be important.
End of answer
So far we have looked at techniques that enable the structures of the different tissues or organs to be imaged in detail. Abnormalities such as constrictions, growths and areas of disease are thrown into relief and the site for treatment can be pinpointed accurately.
An alternative approach to imaging is to monitor the functioning of a particular organ or tissue – that is, to look at it whilst it is working. One method is to monitor a property of something that is normally in the body; for example the circulation of blood can be monitored (using MRI) using the difference in magnetic properties of the iron atoms in oxygenated and deoxygenated haemoglobin. This technique is known as functional magnetic resonance imaging (fMRI). A second approach is to use a traceable agent, such as a radioactive nuclide, that is taken up during the functioning of the organ. Radioactive nuclides can be injected and their progress through a particular tissue monitored by their emission. Techniques using this approach include single photon emission computed tomography (SPECT) and positron emission tomography (PET).
Functional imaging has been used, for example, to explore the action of the brain as it responds to different stimuli. It has typically been used in research experiments whose aim is to map sensory, motor and cognitive functions to distinct anatomical regions of the brain.
The following video shows one of these techniques in practice, PET, to monitor the flow of blood to different regions of the brain while a volunteer is performing a specific task.
In this example, how is PET used to monitor the flow of blood to the brain?
A radioactive isotope of oxygen attached to water is injected into the bloodstream. By monitoring the oxygen, PET monitors the blood flow to different regions of the brain while the volunteer is performing a task.
End of answer
So, how does the ability to monitor changes in the flow of blood enable imaging of the brain?
We need to consider the effect of neuronal activity on metabolic change.
The precise nature of the coupling between neuronal activity and the types of local metabolic change that can be detected is still an issue of some controversy. Nonetheless there is a strong view that there is a relationship and that these techniques can be taken to be sensitive to activity in the brain that correlates with neuronal activity.
The metabolism of the brain is highly complex, but there are certain key chemicals such as glucose and oxygen, which have to be present. It turns out that when a region of the brain is ‘working hard’ there are local changes in the brain’s metabolism, which are associated in some way with the energy requirements of the enhanced neuronal activity. The effects on the metabolism include increases in the amount of blood flowing to the region, changes in the oxygen content of the blood and changes in glucose consumption.
Early PET investigations produced some surprising results. Although functionally induced local increases in blood flow and alterations in the rate of glucose consumption were observed, it was found that oxygen concentrations did not decrease as might be expected, but rather increased. In other words, the enhanced supply of oxygen due to the increased blood flow was more than the local demand. The reasons for this behaviour are still not fully understood. However, the oversupply of oxygen, which is sufficiently large to be detectable, turns out to be very useful for functional imaging purposes.
As mentioned above, fMRI is used to monitor the circulation of blood using the difference in the magnetic properties of the iron atoms in the oxygenated and deoxygenated haemoglobin.
One of the big advantages of fMRI research is that it can be carried out using the modern clinical MRI scanners that are installed in large hospitals. This means that the cost of fMRI can be relatively low compared to other functional imaging research techniques as it can be shared with clinical partners.
The key aim of fMRI is to highlight those areas within a conventional MRI scan of the brain that show activity in response to some form of stimulus. This aim can be realised using several techniques (the level of sophistication is increasing rapidly) but one of the most important is based on the detection of local increases in blood flow in activated areas of the brain.
What did PET show happens to the concentration of oxygen in these areas?
The concentration of oxygen increased although consumption of oxygen also increased.
End of answer
Oxygen is transported around the body in the bloodstream in the form of oxyhaemoglobin. Oxyhaemoglobin and deoxyhaemoglobin are chemically different and also differ in their magnetic properties.
What would you predict for the magnetic moment of deoxyhaemoglobin?
Deoxyhaemoglobin contains Fe2+ coordinated to five nitrogens, all weak-field ligands. This is a d6 ion high-spin system so will be expected to have a magnetic moment of 4.90 µB corresponding to four unpaired electrons.
End of answer
Deoxyhaemoglobin is paramagnetic, so will affect relaxation times in the same way as paramagnetic contrast agents. Oxyhaemoglobin, as you saw in Chapter 8, is diamagnetic.
Under conditions of normal blood flow in the brain, there will be a particular ratio of the oxy to deoxy forms of haemoglobin in the blood (Figure 9.10a) and in an appropriately constructed MRI scan the tissue will appear in the image with a certain brightness. However, as we saw, the oxyhaemoglobin content of the blood in the veins increases after increased brain activity and this causes the local concentration of deoxyhaemoglobin to be correspondingly decreased (Figure 9.10b).
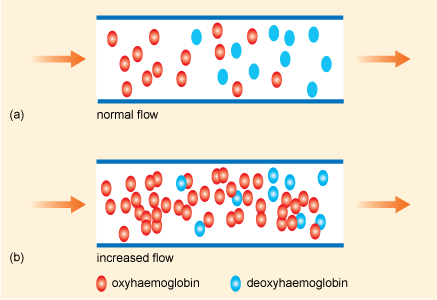
Figure 9.10 Schematic views of the oxyhaemoglobin and deoxyhaemoglobin content of blood during (a) normal and (b) increased flow in the brain.
Source: Cohen and Bookheimer, 1994.
Under the conditions of the pulse sequence used, the tissue will now appear relatively brighter in the image. This is referred to by the acronym BOLD which stands for blood oxygenation-level dependent contrast. Blood makes up a small fraction, typically less than 7%, of the total mass of the brain. The local signal changes that occur in fMRI during activity in the brain are small; however, they are detectable. fMRI also has applications in visualising other areas of function of the human body such as those involved with the cardiac cycle or metabolic activity in tissue.
Extremely fast scanning rates are required for fMRI and measurable changes can be detected, typically of the order of a second, although in very sensitive experiments better than this can be achieved. Resolution of different features can be achieved to about 3 mm apart and it is becoming feasible to record and display what is going on in a volunteer’s brain on a timescale of a few seconds (Figures 9.11 and 9.12).
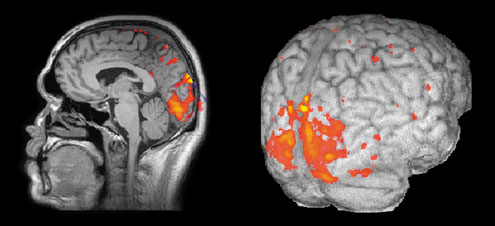
Figure 9.11 The results of an fMRI experiment in which a subject is shown a simple moving visual stimulus. Statistical analysis is used to locate those regions in the brain that show a significant change in image intensity. These results are then superimposed, in the form of a colour overlay, on high-resolution structural images to give better definition of anatomical location.
Source: Dr Krish Singh, Aston University.
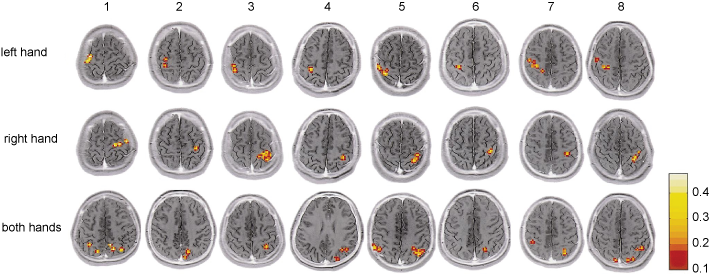
Figure 9.12 Functional magnetic resonance imaging (fMRI) of the human brain through the horizontal plane showing the activity of different brain structures during piano playing tasks for eight subjects labelled 1 through to 8 (grey indicates no changes in activity, red indicates moderate increased activity and yellow indicates high increased activity). In the top row, the images were obtained while the pianist used the left hand only; in the middle row, the right hand only; and, in the bottom row, both hands. For this experiment, subjects were asked not to look at the keyboard to engage only those brain structures activated by motor tasks and not those activated by visual stimuli. Note that for each section, the left hemisphere is represented on the right and the right hemisphere on the left.
Source: Kosuke et al., 2001.
Now watch the following video sequence which covers the use of fMRI in brain research (concentrating largely on psychology rather than imaging itself). This video was made in 2002 and illustrates the potential of the technique, but note that there have been advances since then.
In nuclear medicine, radioactive nuclides (or isotopes) can be used both in diagnosis and in therapy – a topic taken up in Section 9.14. High-energy ?-ray emitters are used for diagnostic scans whereas the cell-damaging properties of ß–- (or a-) emitters make them suitable for cancer therapy. Like fMRI, these diagnostic scans are designed to show the physiological function of an organ in the body being investigated. Box 9.2 summarises the basic processes leading to nuclear decay and emissions.
Radioactivity was first observed by Henri Becquerel in 1896. Nuclear decays are spontaneous changes that occur in radioactive isotopes, causing individual unstable nuclei to transform from one type to another. Photons and particles may both be emitted as a result of these decays, carrying away energy in the process.
A nuclear decay is a random event, and when it will happen can only be predicted statistically. Over a period of time, we can say how many nuclei will decay on average. Radioactive decay is exponential:
N(t) = N0 exp(–?t)
where N(t) is the average number of parent nuclei at time t, N0 is the number of unstable nuclei at t = 0 and ? is the decay constant, and is characteristic of a particular decay process. The half-life, t½ is the time taken for the number of parent nuclei to fall to half t½ = ln 2/?). The types of nuclear decay are described briefly below.
An alpha-particle (a-particle) is the same as a helium nucleus,  , with mass number A = 4 and atomic number Z = 2. It consists of two protons and two neutrons, and so carries two positive charges.
, with mass number A = 4 and atomic number Z = 2. It consists of two protons and two neutrons, and so carries two positive charges.
The uranium-234 nucleus, for example, undergoes alpha-decay to produce an isotope of thorium, with 90 protons and 140 neutrons:
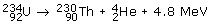
Both electric charge and mass number are conserved and 4.8 MeV of energy is liberated at the same time. This takes the form of kinetic energy and is carried away almost exclusively by the alpha-particle. Alpha-particles are strongly absorbed in tissue (usually within 0–1 mm), they are highly charged, cause cell death and so are not used in diagnostics. Some a-emitters are used in radiotherapy.
Negative beta (ß–)-decay involves the emission of an electron from the nucleus of an atom. It has the effect of converting a neutron to form a proton and an electron. The resultant (positively charged) atom now has a nucleus with one extra proton and so is transformed to a different element; the parent nucleus is said to produce a daughter nucleus. For example 99mTc is produced by the decay of 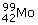 at the same time creating another particle, with zero electric charge, called the electron antineutrino
at the same time creating another particle, with zero electric charge, called the electron antineutrino 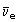 An electron and an electron antineutrino are always created in negative beta-decay. The overall decay process is therefore:
An electron and an electron antineutrino are always created in negative beta-decay. The overall decay process is therefore:
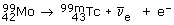
These ß–-particles have a range of up to 10 mm and so are suitable for diagnostic applications. ß–-emitters are also used in radiotherapy to kill cancer cells.
There is a very closely related process, called positive beta (ß+)-decay, in which a positron (i.e. an antielectron) is created, along with an electron neutrino, 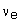 , which has zero charge. In this process, a proton in the original nucleus transforms into a neutron, so decreasing the atomic number by one. An example of a nucleus that undergoes positive beta-decay is the unstable fluorine isotope
, which has zero charge. In this process, a proton in the original nucleus transforms into a neutron, so decreasing the atomic number by one. An example of a nucleus that undergoes positive beta-decay is the unstable fluorine isotope 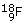 , which transforms into a stable oxygen isotope
, which transforms into a stable oxygen isotope 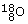 . The decay here can be represented as:
. The decay here can be represented as:
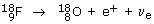
Here, the symbol e+ is used to represent the positron.
ß+-emitting nuclei are used in positron emission tomography, PET.
Gamma (?)-decay occurs when a nucleus is in an excited state; the quantum jump down to a lower-energy state with the same number of neutrons and protons, results in the emission of gamma-ray (?) photons with energies around 1 MeV (1 MeV = 108 kJ mol–1), greater than X-rays. Excited states of nuclei may be created as a result of other decay processes (alpha- or beta-decay) or by the collisions of nuclei at high kinetic energies. For example a metastable form of 99Tc, labelled 99mTc, used in medical imaging, decays to its ground state with the emission of a gamma-ray photon of 142 keV:
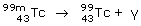
These ?-rays interact only weakly with tissues and are suitable for diagnostic applications.
You should read the extracts in the following Activity from the RSC eBook, Fundamental Toxicology for Chemists, edited by J.H. Duffus and H.G.J. Worth (Duffus and Worth, 1996), which looks at some of the issues of using radionuclides.
Read Sections 19.3, 19.5, 19.6, 19.9, 19.10 of Fundamental Toxicology for Chemists, edited by J.H. Duffus and H.G.J. Worth (Duffus and Worth, 1996), pages 199–204 and 209–212. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.8
There are two radioactive isotopes of iodine that are used in medicine:
123I has a half-life of 13.3 h and emits ?-rays with photon energies of 159 and 529 keV. 131I has a half-life of 8.02 days and emits ?-rays (364 keV) as well as ß–-particles (607 keV).
For what applications are these isotopes likely to be used?
As a ?-emitter 123I is used for imaging. 131I, although used for diagnostics in the early days, is currently mainly used for therapy as it emits ß–-particles as well as ?-rays.
End of answer
The radionuclide introduced into the body is part of a complex molecule known as a radiopharmaceutical. The radiopharmaceutical consists of a radionuclide together with a biologically active molecule. In the presence of disease, the radiopharmaceutical will be distributed selectively around the body and/or will be processed abnormally in the organ or gland under investigation. The radiopharmaceutical complexes are chosen especially for the chemical properties that allow them to interact with the chemistry of the diseased organ, either being preferentially taken up or preferentially excluded.
A typical diagnostic study would involve the administration of the radiopharmaceutical into the body by intravenous injection in liquid, inhalation of a gas or aerosol, or eating a food containing the emitter. The radiopharmaceutical generates an internal (relatively short-lived) radiation source which can be detected externally. Radionuclide imaging techniques are commonly used for bone, heart, kidney, brain, thyroid, liver and other scans, and can easily detect abnormalities such as tumours and abscesses. The technique has the advantages of low cost and easy availability. The disadvantages are that it is of relatively low resolution and is, of course, invasive (as indeed are all contrast agents), particularly if you consider that a radioactive substance is injected into the body. There are two main techniques used in functional imaging using radioactive nuclides (also referred to as radionuclide imaging). In the first of these, PET (positron emission tomography), the radionuclide is a ß+-emitter such as 18F. The compound containing the radionuclide is administered and after a few minutes will have been taken up preferentially by particular sites in the body. Emitted positrons react with electrons in the vicinity of the nuclide to destroy both electron and positron (annihilation) and produce two ?-rays. These two ?-rays are emitted in opposite directions and this defines a line along which the radionuclide must lie. By combining results from many annihilation events, the position of the radionuclide can be determined. Although some metal radionuclides (e.g. 68Ga, 82Rb or 86Y) can be used for PET, the typical radionuclides are 18F and 15O, (we saw an example of the latter in the video in Section 9.7), and we will not consider this technique in any further detail.
In the other technique, single photon emission computed tomography (SPECT), a compound containing a ?-emitting radionuclide is used. Note that in contrast to the ß+-emitter used in PET, ?-emitters only produce one ? photon per nucleus. The emitted ?-ray radiation is detected using an NaI scintillation crystal (a gamma camera) (Figure 9.13), and both the intensity and the position of the radiation can be measured. Increased physiological function, such as that due to a fracture in bone, typically results in an increased concentration of the radiotracer and hence greater intensity. As in the CT scan for X-rays, a computed tomography technique can be used to reconstruct an image of a ‘slice’ through the patient at a particular position by acquiring a series of images from a rotating ?-ray camera.
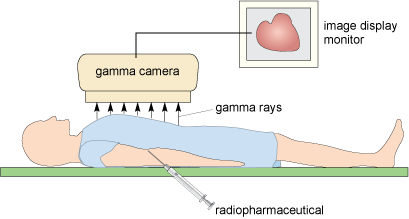
Figure 9.13 Gamma camera set-up, showing the patient close to the camera and the emitted gamma rays forming an image.
Figure 9.14 shows an example of a combined CT scan and SPECT image using 67Ga. The radionuclide is preferentially taken up by infected tissue and clearly shows the spread of the infection in the patient.
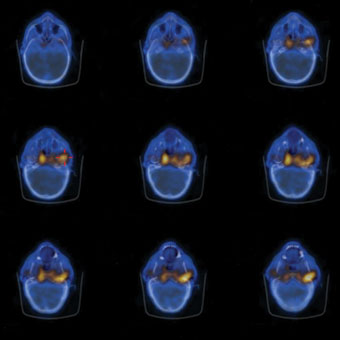
Figure 9.14 A series of combined axial CT scans (in blue) and radionuclide images using 67Ga is shown in Figure 9.14 (in yellow). In this image the gallium is preferentially taken up by infected tissue (in this instance a malignant ear infection which can spread to the bones of the skull).
Source: University Hospitals Coventry and Warwickshire NHS Trust.
The ?-ray emitters used medicinally for imaging are listed in Table 9.4. 99mTc is the most commonly used radionuclide for imaging.
Table 9.4 ?-ray emitters used for SPECT imaging.
| Radionuclide | Photon energies/keV | Half-life |
|---|---|---|
| 99mTc | 140 | 6.0 h |
| 111mIn | 170 and 247 | 2.8 days |
| 67Ga | 93, 185 and 300 | 3.26 days |
| 201Tl | 135 and 167, with characteristic X-rays at ˜80 | 3.04 days |
| 123I | 159 ( and 529) | 13.3 h |
These radionuclides are derived from fission processes in nuclear reactors, by collision of charged particles in cyclotrons, or by taking advantage of natural decay processes in small dedicated generators. This is considered in the following Activity.
Read Sections 3.3.2.2 and 3.3.2.3, pages 136–141 of Medical Applications of Coordination Chemistry (Jones and Thornback, 2007). The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.9
99mTc is readily made in a 99Mo–99mTc generator. 99Mo is a by-product from the nuclear industry as a fission product of 235U:
235 U + 1 n(thermal) ? 99 Mo + 134 Sn + 3 1 n
The 99Mo slowly decays to the metastable 99mTc and then to non-radioactive ruthenium:
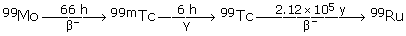
How much 99mTc is left in the body after 24 h?
The half-life is 6 h, so after 6 h, 50% is left, after 12 h, 25%; after 18 h, 12.5%; and after 24 h, 6.25% is left.
End of answer
The generator is loaded with molybdate(VI) [99MoO4]2–, obtained from a nuclear reactor, on to an alumina column. The molybdate slowly decays with a half-life of 66 h (2.75 days) to give the technetate(VII) (pertechnetate) [99mTcO4]–.
The [99m TcO4]– is eluted from the column with saline solution as Na[TcO4]. Appropriate ligands are added, along with a reducing agent (often stannous chloride) to make the desired complex, which is then injected into the patient. As you will see in the following video, 99mTc radiopharmaceuticals have to be prepared in sterile environments, in high purity and yield, rapidly and with minimal manipulation by hospital staff.
Why does a lead-covered syringe offer protection to the nurse giving the Tc injection?
Lead is a heavy element with atomic number 82 and therefore is a strong absorber of ?-rays. It is also readily available and relatively cheap.
End of answer
Technetium is a second row transition metal in Group 7. It is the only d block element that has no stable isotopes. Although it is not naturally occurring, plenty of Tc is available as a by-product from the nuclear industry (over 160 000 kg, which is more than that of the naturally occurring Re from the same Group). Tc can adopt a large range of oxidation states from –1 (d8) to +7 (d0) and coordination numbers vary from 4 to 9. In high oxidation states Tc forms very strong bonds to oxygen.
99mTc is ideal for diagnostic imaging as it emits 140 keV ?-rays and does so with high efficiency. These ?-rays are easily detected by hospital ?-ray cameras.
Once generated, 99mTc has a short 6-hour half-life. Technetium forms stable complexes with a wide range of ligands containing diverse donor atoms (O, N, S, P, As, Cl, etc.), the choice of which depends on the organ to be investigated. For example technetate(VII) can substitute for iodide in the Na/I symporter (NIS) channel in cells of the thyroid gland, so 99mTc-technetate(VII) is used for nuclear medicine imaging of the thyroid gland. The half-life is sufficiently long to synthesise the radiopharmaceuticals, purify them, inject them into a patient and obtain an image, but is short enough to minimise the radiation dose to the patient.
99mTc decays to a daughter isotope, 99Tc, which is a ß–-emitter with a long half-life.
Why is a long 99Tc half-life beneficial here?
ß–-emitters cause cell damage, but because of the long half-life the nuclide is eliminated from the body before it decays and emits a damaging ß–-ray.
End of answer
As you saw in the video the 99mTc can be extracted easily from the generator. Each generator, despite containing only a few micrograms, can be used for the potential diagnosis of many patients.
How long will it take before the 99Mo in the generator is nearly all consumed?
The half-life is 66 h, so 50% will have decayed in 66 h, 75% in 132 h, 87.5% in 198 h and 93.25% after 264 h. Thus the generator will be producing 99mTc strongly for over a week.
End of answer
The following video shows the use of the 99mTc radionuclide to examine the supply of blood to the lungs. (A second radionuclide, 81mKr, which has a half-life of 13 s, is used to study the ventilation of the lungs simultaneously.)
Which ligand was used in the video to monitor the blood supply to the lungs? Why might this ligand have been chosen?
The Tc was reacted with a form of albumin. Albumin, you may remember from Chapter 4, is a transport protein in the blood. The particles tend to become lodged in the small capillaries of the lungs.
End of answer
An alternative to labelling blood cells with the radionuclide is to design a radiopharmaceutical using coordination chemistry, the choice of ligand depending on the organ to which it will be delivered. Different ligands are used for imaging different organs. There are two main types of radiopharmaceutical: Tc-essential and Tc-tagged. In Tc-essential, technetium is an integral part of the drug and the coordination chemistry of technetium plays a significant role – the biodistribution and targeting capability of the imaging agent depend on the lipophilicity (i.e. fat soluble as opposed to water soluble), size and charge of the technetium complex. Tc-tagged are radiopharmaceuticals where the Tc has been joined to a biomolecule via a linker. We will consider these in turn in the next few sections, starting with Tc-essential radiopharmaceuticals. Some of the commercially available Tc-essential radiopharmaceuticals that we will discuss in the next few sections are shown in Table 9.5.
Table 9.5 Some commercial 99mTc radiopharmaceuticals and their primary uses (structures are shown in the text).
| Radiopharmaceutical | Trade name | Primary uses |
|---|---|---|
| 99mTc bicisate (ECD) | Neurolite® | brain imaging |
| 99mTc exametazine (HMPAO) | Ceretec® | brain perfusion imaging |
| 99mTc oxidronate (MDP) | Osteoscan® | bone imaging |
| 99mTc sestamibi | Cardiolite® | heart imaging |
| 99mTc tetrofosmin | Myoview® | heart imaging |
In Section 9.7 we met the use of PET in functional imaging of the brain. Brain imaging radiopharmaceuticals can be used to detect irregular blood flow in the brain due to stroke, brain damage, and Alzheimer’s disease for example. A SPECT image for a patient suffering Alzheimer’s disease is shown in Figure 9.15.
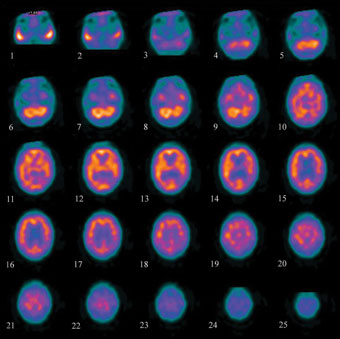
Figure 9.15 Axial view of segments through the brain for a patient with Alzheimer’s disease, taken using the radiopharmaceutical Ceretec®. The brighter the colour, the greater the blood flow. The blood flow in this patient is reduced compared to a patient without Alzheimer’s disease.
Source: University Hospitals Coventry and Warwickshire NHS Trust.
In brain imaging it is essential that the imaging agent must be able to cross the blood–brain barrier (BBB). This requires low molecular mass, a neutral charge and moderate lipophilicity – it has to be lipophilic enough to cross the BBB, but not too lipophilic otherwise it can bind to proteins thus preventing crossing of the BBB. The imaging agent needs to then get ‘stuck’ in the brain to allow enough time for a scan.
The most common 99mTc brain imaging drugs are Ceretec® (9.5) and Neurolite® (9.6).
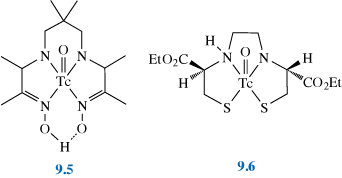
What main structural feature do they have in common?
They are both five-coordinate, square-pyramidal complexes. Ceretec® is coordinated by one oxygen and four nitrogens (from the ligand hexamethylpropyleneamine oxime, HMPAO). Neurolite® is a square-pyramidal mono-oxo, N2S2 chelate complex (with a ligand known as bicisate or ethyl cysteinate dimer, ECD). Both are also complexes of Tc(V).
End of answer
The two compounds use slightly different mechanisms to trap the drug in the brain. Ceretec® reacts in the brain to form a hydrophilic species (whose identity is unclear) that cannot diffuse back out of the brain. It has limited stability in solution. Whereas enzymatic hydrolysis of one of the ester groups of Neurolite® (by an esterase enzyme inside the brain) gives a hydrophobic complex, which causes the compound to become trapped. Similar enzymatic conversions can also occur in the blood as the drug circulates in the body. This impairs brain uptake but allows removal of Tc from non-target tissue via the kidneys.
The following video demonstrates a SPECT procedure to look at the blood supply to the heart under stress and at rest. (The radiopharmaceutical used is technetium labelled tetrofosmin or Myoview®.)
In early days thallium, 201Tl, was used for heart imaging, taken into cells in the heart via the ATP-driven Na/K pump (Chapter 4).
Tl+ is a similar ionic size to K+. Why does this make Tl+ a suitable choice for this mechanism?
Tl+ can replace K+.
End of answer
A series of images of a heart under stress and at rest, using 201Tl is shown in Figure 9.16.
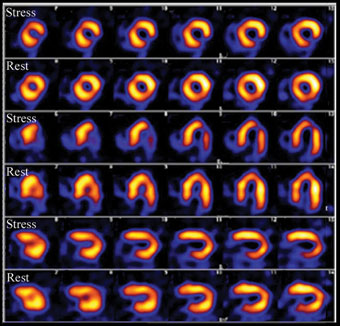
Figure 9.16 Imaging scan of an abnormal heart under stress and rest (3 different views): the brighter the colour the greater the blood flow. You can clearly see that the blood flow under stress is reduced compared to the image at rest.
Source: University Hospitals Coventry and Warwickshire NHS Trust. Hospitals Coventry and Warwickshire NHS Trust.
Thallium(I) is extremely poisonous – how much of an issue is this likely to be in the use of this element in radiopharmaceuticals?
This is not a significant issue as the amounts of radionuclide used are very small.
End of answer
201Tl has a half-life of 3.04 days (Table 9.4). Is this favourable for a radiopharmaceutical?
No, this is rather long compared to the 6 h half-life of 99mTc which is a disadvantage, as it will affect the intensity of the gamma radiation.
End of answer
The length of half-life will affect the number of radioactive decay events observed in a given time and therefore the intensity of the images obtained from the scintillation camera. Ideally we need an imaging agent that does not decay too rapidly, so that there is a reasonable amount of activity still remaining once it has reached the required target organs. It should also clear the body efficiently. In the time taken for the reagents to leave the body, any daughter nuclei should either be stable or have half-lives of sufficient length to ensure that no harmful secondary reactions can take place.
201Tl, as well having a fairly long half-life, also has a disadvantage of low photon energy and has since been displaced by technetium complexes.
The technetium complex first used for heart imaging was [Tc(DMPE)2Cl2]+ (see structure 9.7) but this has now been superseded by Myoview® (9.8) and Cardiolite® (9.9).
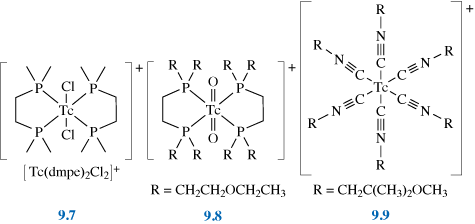
What are the oxidation states of Tc in these three complexes?
[Tc(dmpe)2Cl2]+ is Tc(III) – it has two Cl– ions and a positive charge; Myoview® is Tc(V) – it has two = O bonds and a positive charge; Cardiolite® is Tc(I) – it has uncharged isonitrile ligands and a positive charge.
End of answer
Clearly, oxidation state is not a determining factor.
What features do these three complexes have in common?
They are octahedral, carry a single positive charge and the metal is coordinated by strong-field ligands.
End of answer
The octahedral coordination helps prevent reaction with the metal and thus aids stability, as does the use of strong-field ligands. The single positive charge means that the complexes are both soluble in water (and therefore in blood) but are sufficiently lipophilic to pass through the membrane enclosing the heart muscle. It was found that only cationic complexes were effective for heart imaging, and it is thought that the positive charge is attracted to the mitochondria in the cells, thus helping to trap the complex.
The dmpe complex was found to reduce in vivo to a neutral Tc(II) complex, which is washed out from the heart to the liver. This led to the development of Myoview® and Cardiolite® (9.8 and 9.9), which have a longer lifetime in the heart as they are more resistant to reduction. As isonitriles are very smelly, it took a great deal of experimentation to find an effective ligand that was also acceptable to patients.
Myoview® is easily prepared (90% yield) from [99mTcO4]– + SnCl2 + diphosphine.
What is the oxidation state of Tc in [99mTcO4]–?
[99mTcO4]– contains Tc(VII).
End of answer
What is the role of SnCl2 in this reaction?
SnCl2 will behave as a reducing agent, reducing Tc from Tc(VII) to Tc(V) and itself being oxidised to Sn(IV).
End of answer
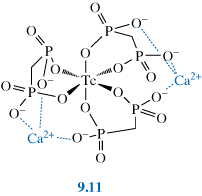
99mTc complexes of phosphonate ligands, especially methylene diphosphonate, MDP (9.10), have been developed for bone imaging.

The technetium complexes of these ligands have many possible structures depending on the pH, temperature and concentration. Most contain technetium in oxidation state +4.
As we saw in Chapter 6, bone is effectively calcium phosphate (hydroxyapatite). Osteoscan®, a Tc–MDP complex (9.11), has a high affinity for the Ca2+ in the hydroxyapatite, possibly due to two tridentate binding sites for Ca2+.
Stressed bone, such as a fracture (or arthritis), has higher concentrations of Ca2+ ions, which show up as ‘hot spots’ on a scan as seen in Figure 9.17.
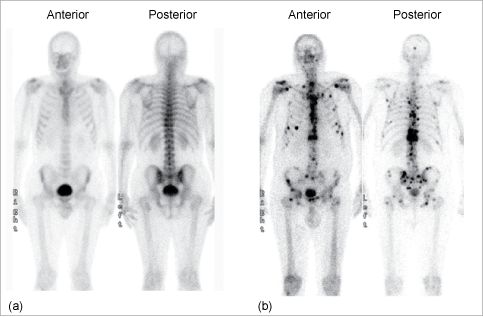
Figure 9.17 Bone scans with 99mTc. (a) A normal scan; the skeleton can be seen clearly on both front (anterior) and back (posterior) views, and there are no unexpected ‘hot spots’. (b) A scan showing bone metastases; in addition to the skeletal outline there are regions of high radioactivity which indicate greater metabolic activity at the metastases. Note that the bladder shows up as radioactive; this is because the radionuclide is removed from the body via the kidney and bladder.
Source: University Hospitals Coventry and Warwickshire NHS Trust.
The various imaging agents so far described are based on traditional coordination compounds. Much work has been carried out recently into improving the targeting of Tc to the required organs. This can take two different approaches. We can either use ligands for Tc that more closely resemble natural bioligands or attach Tc compounds to genuine biomolecules.
The targeting biomolecule (e.g. antibody, peptide, hormone) is attached via a linker unit to a chelated 99mTc metal centre, and the 99mTc is carried by the biomolecule to the target site as a passenger. Figure 9.18 shows a schematic structure for the tagged complex. The choice of biomolecule depends on the target site. Hence these imaging agents have a very high specificity and affinity for the target sites. Activity 9.10 explores this in further detail.
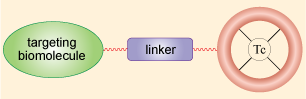
Figure 9.18 Schematic diagram of a tagged radiopharmaceutical.
Read Sections 3.3.12 and 3.3.12.1, pages 185–189 of Medical Applications of Coordination Chemistry (Jones and Thornback, 2007) and then answer the questions below. The complete book can be accessed through the RSC eBook collection, and this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.10
What is the Tc metal centre typically used in these radiopharmaceuticals.
Many of the tagged radiopharmaceuticals in the extract contain Tc(V), as TcO3+.
End of answer
What factors are important in choosing an appropriate linker for these tagged radiopharmaceuticals?
The link between the targeting biomolecule and Tc metal centre needs to be stable under physiological conditions. In addition, a simple preparation route for the tagged pharmaceutical is desirable which will influence the choice of linker.
End of answer
Many different chelators have been studied, with different donor atoms, coordination numbers, geometries, solubility, hydrophilicity, charge, macrocyclic and non-macrocyclic, etc.
The kinetics of the reactions that the drug undergoes once in the body can also be altered by modifying the linking group. For example, introduction of poly-aspartic acid groups can make the imaging agent much more hydrophilic.
Examples of tagged radiopharmaceuticals include radiopharmaceuticals that bind to the oestrogen receptor. These can be used to monitor the progression of breast tumour therapies for 60–70% of breast tumours, treated with agents such as tamoxifen that also bind to the oestrogen receptor sites.
Similarly most prostate cancers are androgen and progesterone receptor positive and can be imaged with the appropriately labelled receptor hormones.
Figure 9.19 shows a targeting molecule which is made from a progesterone derivative, linked by a phenyl group to a technetium complex chelated by an N2S2-based ligand. Attachment of the linker molecule to the steroid takes place at 11ß site and does not impair receptor binding.
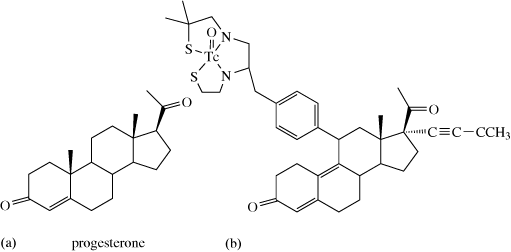
Figure 9.19 (a) Progesterone and (b) Tc-tagged progesterone.
Several diseases, for example, epilepsy, Alzheimer’s and Parkinson’s, and a variety of psychiatric conditions are associated with changes in the density of neurotransmitter receptor sites in the brain. These can be monitored and studied with Tc-tagged drugs.
For example, as cocaine (9.12) and its analogues block dopamine transporter sites, cocaine-derivatised Tc imaging agents can be used to monitor the progress of Parkinson’s disease.
One example is Tc-TRODAT (9.13), a cocaine derivative linked via the seven-membered ring to a Tc(V) oxo-core through an N2S2 ligand. A related iodine-labelled radiopharmaceutical used to image the brain, also based on a cocaine derivative, is discussed in Box 9.3 which considers one of the most common nuclides used for imaging, 123I.
One of the most common nuclides used in imaging is 123I, and although not a metal we will consider it briefly here.
123I has a half-life of 13.3 h and emits ?-rays with photon energies of 159 and 529 keV. It is prepared in a cyclotron.
123I has been used in thyroid imaging. Radioactive iodine is actively concentrated or trapped by the thyroid gland and is subsequently incorporated into thyroglobulin, an iodine-containing glycoprotein that is a precursor for the thyroid hormones found in the thyroid gland. ([99mTcO4]– is also concentrated in the salivary glands and gastric mucosa and can be used in thyroid imaging, although it is not incorporated in thyroglobulin.)
A number of 123I radiopharmaceuticals have been used for brain imaging, as in the case of the Tc-tagged radiopharmaceuticals used to image the brain, these target the dopamine transporter sites in the brain. An example includes 123I ß-CIT or DOPASCAN® (9.14). Figure 9.20 compares the results of an MRI scan obtained using this radiopharmaceutical for a healthy brain and one with Parkinson’s disease. Recent clinical trials suggest that images obtained using DOPASCAN® may aid in the diagnosis of Parkinson’s disease and Progressive Supranuclear Palsy.

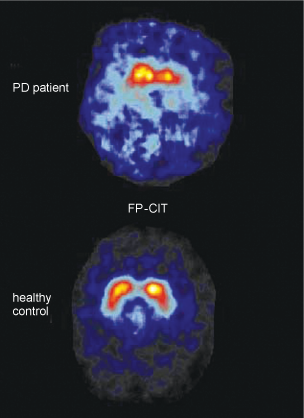
Figure 9.20 MRI scan with DOPASCAN® (top) in a patient with Parkinson’s disease and (bottom) a healthy human control subject.
Source: Morgan and Nowotrik, 1999.
Monoclonal antibodies (MAbs) are proteins (molecular mass 105–106), which are produced naturally by the body to combat disease/cancer, and have a very high affinity for specific sites. They can be produced by introducing human cancers into mice, causing the mice to produce the antibody specific to the cancer. The mouse cells making the antibodies are then extracted and cloned to give a source of MAbs.
The large size of antibodies, however, can mean that they are slow to distribute throughout the body, so antibody fragments have been developed which retain the specific binding characteristics of the antibody but have a much lower molecular mass.
Several Tc-tagged antibody fragments are already available commercially for diagnostic applications in colorectal cancer, osteomyelitis and diabetic foot ulcers.
These imaging agents can often detect changes in the body before any clinical symptoms are noticed (scans can also be positive up to 5 months before abnormalities show on a normal CT scan) and can hence be crucial for early diagnosis.
This survey has provided an introduction to the advances in medical imaging that have been made possible by applying the underlying principles of coordination chemistry to biological systems. We will now turn our attention in the rest of this chapter to the ways in which metallopharmaceuticals can directly aid in the fight against disease, as therapeutic agents.
Metals, their salts and complexes are used in therapy for a number of different applications, as was described briefly in the introduction to this chapter (albeit mainly from an historical perspective). Table 9.6 lists some of the uses of metal compounds in therapeutic medicine today (expanding on the list in Table 1.3 in Chapter 1).
Table 9.6 Example of metals used in therapeutic and other healthcare applications.
| Metal | Form (common/trade name) | Use/treatment |
|---|---|---|
| Li | carbonate (Camcolit®) | manic-depressive illness |
| Na | bicarbonate (Alka-seltzer®) | heartburn |
| Mg | sulfate (epsom salts) | constipation |
| hydroxide (milk of magnesia) | heartburn | |
| Al | hydroxide (Gaviscon®) | heartburn |
| silicate (kaolin) | diarrhoea | |
| Ca | carbonate | heartburn, peptic ulcer, diarrhoea |
| Ga | Ga(III) complex | cancer |
| As | organic arsenic compound (Melarsoprol®) | sleeping sickness |
| Sr | 89Sr complex | bone cancer |
| Sb | sodium stibogluconate | Leishmaniasis |
| Bi | bismuth subsalicylate C 7 H 5 BiO 4 (Pepto-Bismol ® ) | heartburn, diarrhoea |
| colloidal bismuth subcitrate (De-nol®) | peptic ulcer | |
| Ti | oxide | sunblock |
| V | complex | diabetes |
| Fe | Na 2 [Fe(CN) 5 (NO)].2H 2 O | hypertension |
| Cu | histidine complex | Menkes disease |
| Zn | oxide | sunblock |
| oxide with 0.5% Fe2O3 (calamine lotion) | antimicrobial agent | |
| Y | 90Y complex | bone and liver cancer |
| Zr | Zr(IV) glycinate | antiperspirant |
| Ru | Ru(III) complex | cancer |
| parasitic disease | ||
| Ag | Silver sulfadiazine | burns |
| Pt | Pt(II) complexAg/Hg amalgam | cancerdental amalgams |
| Pt(IV) complex | cancer | |
| Au | Au(III) complex | cancer |
| Au(I) complex | arthritis | |
| Re | 186Re, 188Re complex | bone cancer |
| Ti | Ti alloy | hip and knee replacement |
| Sm | 153Sm complex | bone cancer |
As you can see from Table 9.6 the range of applications and metals used is diverse. Metal salts are used in a number of common medicines and other items that can be bought ‘over the counter’ (Box 9.4), as well as for therapeutic applications taken under medical supervision s. Metals are also used in implants, in bone replacement and in dentistry. We will not attempt to cover all these here in great detail, but rather concentrate on a few in more depth.
Aluminium compounds are commonly used as antiperspirants. They are generally applied as a charged complex of mildly acidic aluminium hydroxychloride in solution.
Why is the aluminium applied as a charged complex?
To keep the aluminium in solution to aid application, typically via an aerosol or roll-on.
End of answer
During perspiration the pH is raised to more neutral pH causing aluminium to precipitate as the insoluble aluminium hydroxide. This forms a plug, blocking the pores in the skin.
Precipitation is also believed to be one of the major roles for bismuth in treating peptic ulcers. Peptic ulcers (the most common are gastric (stomach) and duodenal ulcers) can be very painful, especially when stimulated by gastric and duodenal acids present in the gastrointestinal tract. Many peptic ulcers are associated with the bacterium Helicobacter pylori, a bacterium that thrives in the acidic environment of the stomach. The bacterium causes inflammation by preventing regulation of acid in the gastrointestinal tract. The acidic environment present in the stomach results in precipitation of the bismuth as bismuth oxychloride and/or bismuth citrate polymers. These precipitates coat the ulcer site isolating it from the gastric and duodenal acids and allowing it to heal. Bismuth may also have an antibacterial action, and given in combination with antibiotics is particularly successful at treating stomach ulcers. Bismuth subsalicylate is also sold over the counter as an antacid and to treat diarrhoea.
Magnesium and aluminium hydroxide are further examples of antacids sold commercially to treat heartburn. Heartburn arises with regurgitation of gastric acid up the oesophagus where it produces a burning pain.
Suggest why these compounds might be used as antacids?
These compounds are of course bases. The hydroxide ions neutralise the stomach acid.
End of answer
Other examples of antacids include sodium bicarbonate and calcium carbonate, which are also bases.
Magnesium hydroxide can also be used as a laxative (as are a range of other magnesium compounds). Mg2+ is not readily absorbed by the body and remains in the intestines in faeces where it will absorb water from the surrounding tissue. This results in softening the faeces as well as encouraging excretion as the increase in volume stimulates the intestines. The laxative effect of Mg2+ when given as an antacid can be neutralised by addition of aluminium hydroxide, which is also an antacid but also can cause constipation. In this case the aluminium absorbs water from the faeces.
Zinc oxide is commonly used to treat a variety of skin conditions and is a key ingredient in calamine lotion, barrier and nappy creams to sooth itching and irritation. It is also occasionally used as a sunblock, although TiO2 is more commonly used in this role, acting as a physical block, the particles absorbing UV radiation.
In Section 9.2 we discussed the use of metal supplements to supply metal ions to treat metal deficiency. In particular, we discussed the use of copper histidine complexes for the treatment of Menkes disease. Metals are also used in anticancer drugs, both for chemotherapy and radiotherapy. We will discuss these in some length, starting with platinum anticancer drugs, and the story of the molecule that started it all, cisplatin, before moving on to consider second and third generation platinum anticancer drugs. The section on chemotherapy for cancer will finally consider development of metal anticancer drugs other than those containing platinum. Our discussion of anticancer drugs will then consider the use of metals in radiotherapy. Finally, we will take a brief look at some of the other therapeutic applications of metal compounds.
The most effective drugs for combating certain forms of cancer are a series of platinum-containing complexes. They have transformed the statistics in testicular cancer from a rare chance of survival in the 1970s and before, to around a 90% survival rate today. The discovery of their efficacy is one of serendipity.
The structure of cisplatin, the first of these compounds to be discovered, is shown in 9.15.

It is a square-planar Pt(II) complex, first synthesised in 1825 by Peyrone; its structure was later (1893) characterised by Werner.
In 1965 Barnett Rosenberg, a physicist, noted similarities between the appearance of magnetic lines of force and a cell during the process of mitosis, when the chromosomes in the cell replicate and divide into two identical diploid cells in several stages (Figure 9.21a and b). He wondered if an electric field would affect cell division. He conducted an experiment on the bacterium E.coli, subjecting it to a field in an electric cell containing platinum electrodes, with a growth medium of ammonium chloride for the bacteria. The results were startling – but not what he expected. He found that cell growth was not affected, but that cell division was curtailed with the result that he observed the growth of long filaments (Figure 9.21c and d). He realised that the inhibition of cell division could be a very important discovery for cancer therapy.

Figure 9.21 (a) cell in the process of mitosis; (b) magnetic field; (c) scanning electron microphotograph of normal E. coli (Gram-negative rods); (d) scanning electron microphotograph of E. coli grown in medium containing a few parts per million of cis-diamminedichloroplatinum(II). (Same magnification in c and d.) The platinum drug has inhibited cell division, but not growth, leading to long filaments.
Source: (a) D Marta S. Walters, University of California, Santa Barbara and Professor Spencer W. Brown, University of California Berkley; (b) Physics Part 2. 1960/1962. John Wiley & Sons Ltd.; (c) and (d) Professor Barnett Rosenberg;
Further studies showed that the platinum electrodes react slowly with NH4Cl in the presence of light and an electrical current to give cisplatin, [Pt(NH3)2Cl2]. Early laboratory experiments showed cisplatin to be active against tumours in mice and in 1971 it entered clinical trials. It was finally approved for clinical use in the USA in 1978, and now together with its derivative drugs, accounts for over $2.2 billion in the drugs market. These drugs are used very successfully against testicular and ovarian cancer (> 90% survival rate) but also for head and neck, bladder, lung and cervical cancers and lymphoma, melanoma and osteosarcoma. The following videos (made in 2002) summarise the history and development of these drugs, with interviews with leading players in the field, including Barnett Rosenberg himself. The first clip looks at the experiment that led to the discovery of the anticancer properties of cisplatin.
Before we consider the mechanism of the anticancer effect of cisplatin, we will look at the chemistry of Pt in more detail.
Platinum, Pt, is to be found at the bottom of the third transition series in Group 10. It has the electronic configuration [Xe] 4f14 5d9 6s1. Due to poor shielding by the 5d and 4f orbitals it has a high effective nuclear charge. This results in very high ionisation energy and accounts for the stability of the main oxidation state II, which is not easy to oxidise. Some compounds of Pt(IV) and Pt(VI) are found but they are strong oxidants. The electronegativity of Pt is similar to that of C and P.
Pt(II) is the most common oxidation state. It mainly forms d8 square-planar complexes.
Do Pt(II) square-planar complexes conform to the 18-electron rule?
No, the four ligands each contribute two electrons and with the eight from Pt, they are thus 16-electron complexes.
End of answer
What would you predict to be the magnetic properties of such complexes?
16-electron square-planar complexes are diamagnetic (Figure 9.22).
End of answer
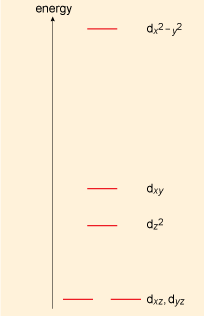
Figure 9.22 Crystal field splitting diagram for a square-planar complex.
Note that some 18-electron trigonal bipyramidal complexes of Pt(II) are also known.
The square-planar complexes PtX2L2 can exhibit cis/trans isomerisation as shown below:
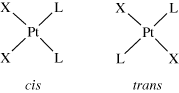
The synthesis of square-planar complexes of platinum is made easier by the observation that some ligands cause the ligands trans to themselves to become labile or more easily replaced. Such ligands are said to exhibit the trans effect. A classic example is the synthesis of the cis and trans isomers of diamminedichloroplatinum(II) as seen in Equations 9.2 and 9.3.
In complexes that contain both chloride and ammonia ligands, whenever a choice is possible, a ligand opposite a chloride ligand, i.e. trans, is replaced in preference to one trans to ammonia. Chloride is said to have a greater trans effect than ammonia.
The trans effect is due to the effect of the opposite ligand on the different rates of the substitution of the different ligands, so is best described as a kinetic effect. The general order of decreasing trans effect is:
CO, H- > PR3, RS > NO2- > I- >Br- > Cl- > pyr > NH3 > H2O
The trans effect is difficult to explain theoretically and we will not attempt to do so here.
With strong oxidants, platinum(II) complexes can undergo oxidative addition to Pt(IV):
Pt(IV) has a d6 configuration. The complexes are usually six-coordinate, 18-electron, octahedral complexes. They are kinetically inert to ligand substitution.
What would you expect to be the magnetic state of octahedral Pt(IV) complexes?
The strong-field (low-spin) complexes will be diamagnetic, as all the t2g orbitals are full. Weak-field complexes will be paramagnetic.
End of answer
Pt(IV) complexes can readily undergo reductive elimination reactions to give Pt(II) complexes. For example:
NMR is extremely useful for elucidating the structures of platinum complexes and 195Pt NMR is 20 times more sensitive than 13C NMR. It produces a large range of chemical shifts (over 10 000 ppm). Platinum has an isotope, 195Pt, with nuclear spin of I = ½. This isotope has an abundance of 33.8% leading to interesting and characteristic splitting patterns. (Box 9.5)
The NMR spectrum for a given nucleus may show ‘satellites’ due to spin–spin coupling with spin I = ½ nuclei of low to medium natural abundance. A well known example involves spin–spin coupling to 195Pt. This is the only stable magnetic isotope of platinum: it has spin I = ½ and a ‘medium’ natural abundance of 33.8%.
The presence of ‘platinum-195 satellites’ is illustrated in Figure 9.23 which shows the ethene part of the 1H NMR spectrum recorded at 23 °C of trans-[PtCl2(CH2=CH2)L] dissolved in CDCl3 where L is an organic ligand. The spectrum consists of just three equally spaced lines in the approximate intensity ratio 1:4:1.
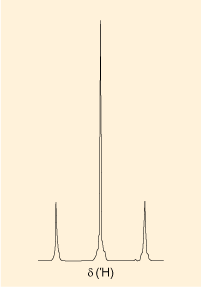
Figure 9.23
The interpretation of the 1H NMR spectrum is simplified because the four hydrogen atoms of the ethene group form a chemically and magnetically equivalent set. Hence, in the absence of any spin–spin coupling, this set will give rise to a single resonance line. This line, in fact, corresponds to the central line in the observed spectrum and it must arise from the 66.2% of molecules of the platinum complex that contain non-magnetic platinum. The remaining two lines in the spectrum (the ‘satellites’) represent a doublet which arises from 1H, 195Pt spin–spin coupling in the 33.8% of molecules that contain 195Pt. The doublet splitting (in Hz) gives the value of 2J(1H, 195Pt). This interpretation is also consistent with the observed intensities of the lines. They would be expected to be in the ratio (33.8/2):66.2:(33.8/2) – which is roughly 1:4:1, as observed. Overall, the observed spectrum effectively represents contributions (a singlet and a doublet) from two different types of molecule when viewed from an NMR perspective.
The 1:4:1 ratio is diagnostic for spin–spin coupling to platinum and is observed in the spectra of both high-abundance nuclei, for example 31P, and low-abundance nuclei, for example 13C. In the latter case, even though 13C is of low natural abundance, a given 13C nucleus in a molecule will still have a 33.8% chance of spin–spin coupling to 195Pt.
Predict the form of the proton decoupled {1H}-15N NMR spectrum of cisplatin.
The two 15N atoms are chemically equivalent and thus produce a single resonance. As the nitrogens are bonded directly to platinum they will spin–spin couple with the 195Pt isotope which is in about 33% abundance. This then produces the characteristic 1:4:1 splitting pattern (Figure 9.24) with Pt satellites.
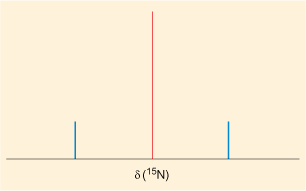
Figure 9.24 Sketch of the predicted form of the {1H}-15NMR spectrum of cisplatin.
End of answer
Having looked at the chemistry of Pt, we will now return to the cisplatin story, focusing on the aquation of cisplatin in the body.
After intravenous administration, the neutral cisplatin complex dissolves in the water of the bloodstream, whence it circulates and passes into the cells, crossing the cell membranes by passive diffusion (Chapter 3).
Is Pt a hard or soft metal? What type of molecules or ions in the bloodstream might react with cisplatin before it gets a chance to cross the cell membrane?
There are many species present in blood including sugars, salt, proteins, oxygen and of course water. Pt is a soft metal, so it is soft bases that pose the greatest threat and also those species that are in the greatest concentration. Thus sulfur-containing compounds, such as cysteine, are a possibility, as is water itself.
End of answer
In practice, the high concentration of chloride ions in the blood suppresses the hydration of cisplatin, and it passes into the cells mostly unchanged.
However, once in the cells, it is a different story as the concentration of chloride is now much lower (4 mmol dm-3 inside compared to 100 mmol dm-3 outside). The cisplatin slowly reacts stepwise with the water in the cells to form first the monosubstituted aqua complex, and finally the disubstituted ion. Reaction 9.6 shows the hydration equilibria involved.
195Pt and 15N NMR studies have shown the mono-aqua square-planar complex to be the active species. This is illustrated in the following clip where Professor Lippard (MIT) describes some of the work completed to help understand the chemistry involved.
How does the charge on the Pt complex change on aquation?
The complex is now positively charged.
End of answer
The positive charge on the substituted complex means that it is attracted to the negatively charged surface of the DNA in the cell. There is a 2–3 h delay in sensitisation after administration of cisplatin due to the slow formation of this substituted complex.
The attraction of cisplatin to DNA was confirmed by treatment of HeLa cells (see Box 9.6) with a high dose of 195mPt radiolabelled cisplatin, which shows where cisplatin binds in the cells. Analyses give:
~ 1 Pt in 104–105 protein molecules
~ 1 Pt per 10–1000 RNA molecules, but
~ 9 Pt per 1 DNA molecule.
It had been found that there is a correlation between Pt–DNA adducts in peripheral blood cells and disease response in cisplatin patients and so Pt–DNA binding has been the main focus of studies.
An HeLa cell is a so-called ‘immortal’ cell line commonly used in cancer research. The cells are said to be ‘immortal’ because under suitable laboratory culture conditions they can divide an unlimited number of times. The cell lines are descended from the cervical cancer cells of Henrietta Lacks, who died from cancer on 4 October 1951. These cells have an enzyme that prevents the shortening of the telomeres (section of DNA at the ends of the chromosome) due to ageing and so are able to go on dividing much longer. These cultures are thus extremely useful, particularly as the growth rate is also very fast, even when compared with other cancer cells.
As it is clear that the DNA in the cell is being targeted by the cisplatin, it is useful to consider which parts of the DNA might be preferentially bound by the metal ion. You can refresh your memory about the molecular structure and function of DNA in Box 9.7.
DNA, deoxyribonucleic acid, is a biopolymer composed of repeat monomers known as nucleotides. Each nucleotide consists of a phosphate group, a sugar molecule and a nitrogen-containing base. The sugar is deoxyribose (Figure 9.25a). There are four different bases in DNA: adenine, guanine, cytosine and thymine, usually abbreviated to A, G, C and T, respectively. Their structures are shown in Figure 9.25b. A fifth base, called uracil, U (Figure 9.25c), usually takes the place of thymine in RNA and differs from thymine lacking the methyl group on its ring.
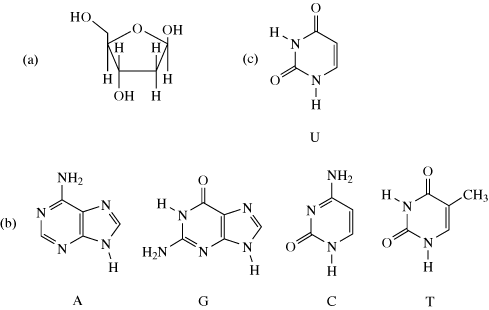
Figure 9.25 (a) The structure of the sugar deoxyribose. (b) The structures of the four DNA bases. (c) The structure of uracil.
DNA has a double-helix structure, which lends it stability. The strand of alternating phosphate groups and sugars with ester linkages forms the sugar–phosphate backbone of each strand, and the bases protrude out from this towards the other strand of the helix. Along the length of the polynucleotide chain, each base makes a specific pairing (Watson–Crick pairing) with a corresponding base in the other polynucleotide chain with hydrogen-bonding: T (thymine) pairs only with A (adenine) and C (cytosine) pairs only with G (guanine). The pairs of complementary bases are thus T and A; and C and G, as shown in Figure 9.26 which shows a portion of a DNA molecule. DNA molecules are by far the largest known molecules in living organisms, some containing millions of nucleotides.
Figure 9.26 A portion of the DNA double helix showing ten labelled complementary base pairs and the positions of the major and minor grooves are indicated.
Inspection of the double-helix structure reveals two grooves (Figure 9.26), the major groove and minor groove, with approximate widths of 220 pm and 120 pm, respectively. The bases are more exposed in the major groove and are therefore more accessible to other molecules and this is where most reactions take place.
DNA can take a number of different conformations, of which three are found in nature, labelled as A–, B– and Z–DNA; of these B–DNA is the form most commonly found in cells.
The obvious targets for the Pt ion would appear to be the nucleotide bases, and we will initially consider the chelating abilities of each base in turn. We will first return to the work of Professor Lippard in the following video clip.
From the NMR studies, to which base does the cisplatin appear to bind preferentially?
The cisplatin binds to the guanine base, coordinated with the nitrogen labelled as N7.
End of answer
Theoretical and experimental studies have shown that the N7 atom on guanine (imidazole) is the most electron rich centre. (Remember that atom numbering starts at the functional group as shown, 9.16.)

The monohydrated cisplatin reacts with DNA to form adducts, mostly forming Pt–N bonds to guanine N7 as shown, 9.17:
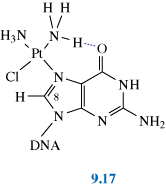
The Pt–G(N7) bond is very stable and can only be broken by a strong nucleophile (e.g. cyanide, thiourea). Possibly (as shown in 9.17) hydrogen-bonding also stabilises the adduct.
This adduct can be readily detected using 1H NMR spectroscopy: the resonance of the C8–H (shown in 9.17) in guanine is a singlet at d 7.8. However, when guanine is complexed to cisplatin at N7, this singlet shifts to d 8.8 and 195Pt satellites are also observed.
This large shift means that 1H NMR becomes a very useful tool in elucidating the structures of these more complex systems – along with the crucial technique of X-ray crystallography as we will see in the next section.
Oligonucleotides are short sections of DNA (2 to 15 nucleotides long) used for model studies; these are also often referred to as a ‘duplex‘ in recognition of the two-stranded helical structure of DNA. Using these, early structural characterisation of DNA-bound cisplatin, using X-ray crystallography, 1H NMR and molecular modelling, showed that cisplatin was found to form a cis complex with two adjacent guanines on the same strand (known as a G(N7)–p–G(N7) linkage, often shortened to G–p–G or G–G), which is shown in Figure 9.27.
Figure 9.27 The NMR structure of the DNA duplex dodecamer,
d(CCTCTG*G*TCTCC. GGAGACCAGAGG), containing the cisplatin (GpG–N7(1), N7(2)) 1,2-intrastrand cross-link at the position of the asterisks. (Based on the pdb file 1a84 (Gelasco and Lippard, 1998).) Jmol version
Experiments found that cisplatin formed several different types of adduct with the DNA oligomers. The major product (60–65%) is the G–G 1,2-intrastrand link between guanine residues which reside in the major groove in the B-form DNA (also shown in Figure 9.27): cis-[Pt(NH3)2(G–p–G)] , 9.18. Smaller quantities of other adducts include:
20–25% of a G–A 1,2-intrastrand: cis-[Pt(NH3)2(A–p–G)], 9.19
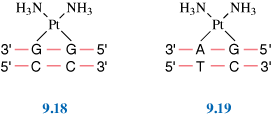
5–10% of more widely spaced guanine adducts: the 1,3-intrastrand and G–p–G interstrand complexes (9.20, 9.21 and Figure 9.28). (Note: N in 9.20 represents another base.) In the latter, cisplatin forms a cross-link between the two strands.
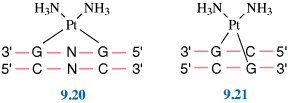
2–5% of a monoadduct, 9.22 and < 1% of DNA–protein binding, 9.23.
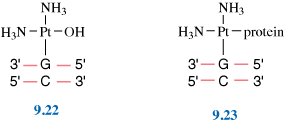
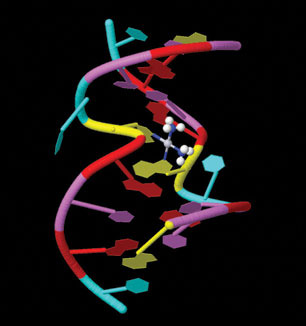
Figure 9.28 The NMR solution structure of the short duplex [d(CAT–AG*CTATG)]2 cross-linked at the position of the asterisks. (Based on the pdb file 1ddp (Huang et al., 1995).) Jmol version
It is thought that the 1,2-intrastrand cross-links are important to anticancer activity as they are the major adducts formed, and clinically inactive compounds, such as the trans-dichlorodiammineplatinum(II) (transplatin) fail to form these cross-links.
The mechanism of the reaction of cisplatin with DNA is shown in Figure 9.29.
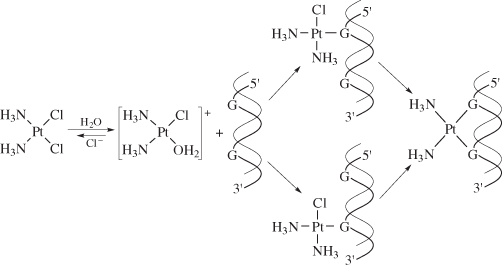
Figure 9.29 Scheme showing the reaction of cisplatin with DNA.
Kinetics are very important in this sequence of steps; the aquation of cisplatin is the slow rate-limiting step and reaction of the cationic Pt complex with the DNA strand is fast.
Hydrogen-bonding from NH3 and OH2 ligands to the phosphate backbone of DNA is possibly important in orientating the Pt complex.
The structural studies of cisplatin binding to oligonucleotides (see previous section) show that different adducts distort the DNA in different ways as discussed in the following video clip.
As you may have noted in Figure 9.27 the Jmol version can be seen again here, the main observed effects of 1,2-intrastrand cross-links are:

This all leads to destabilisation of the duplex, which in turn blocks replication and inhibits transcription. Replication stops at sites corresponding to one nucleotide preceding the first Pt–G residue and at positions opposite the two Pt–G residues (Figure 9.30).
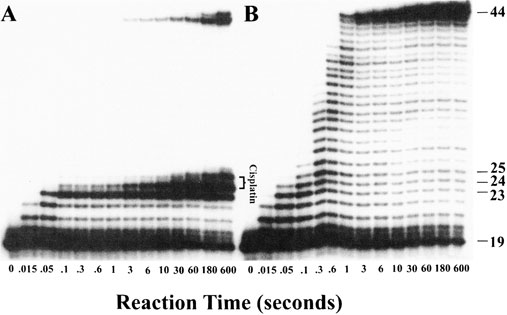
Figure 9.30 Gel electrophoresis data obtained during a kinetic study of the effect of a cis-GG adduct on DNA polymerisation by HIV-1 reverse transcriptase. Panel A shows fragments generated by enzymatic replication of a 44–bp DNA duplex containing a site-specific cis-{Pt(NH3)2}2+ cross-link at G(24)/G(25). Polymerisation is blocked by platination of the substrate. Panel B depicts results for an unmodified DNA probe. (Suo et al., 1999)
There is also some evidence that suggests that cisplatin induces cells to undergo apoptosis (programmed cell death). This occurs because the cell recognises the damaged DNA and triggers the mechanisms that signal the cell to die. This self-destruct mechanism is present in all cells and is part of the organism’s way of destroying cells that might be harmful to the organism. (Similar mechanisms lead to spontaneous destruction of cells carrying harmful mutations and prevent, for example, the full-term development of many fetuses.) See Box 9.8 for information on cell death and DNA repair systems.
Damage is constantly caused to cells and DNA by normal metabolism and by external factors such as UV radiation, smoking, chemicals and so on. Sometimes this leads to the damaged cell swelling and then bursting, a form of cell death known as necrosis. DNA is continually checked for ‘errors’ in its sequence by various proteins. If there is a large amount of damage, then enzymes come into play that trigger the death of a cell by apoptosis or programmed death. If the damage is not too great, then the checking proteins activate enzymes to eradicate the errors and perform a ‘repair’ by excising the damaged part and reconstituting the sequence. Indeed it is the existence of these DNA repair systems that is believed to lead to resistance to cisplatin in certain cancers. On the other hand, studies have shown that repair-deficient mutant cells are much more sensitive to cisplatin.
Professor Lippard talks us through the effect of cisplatin in the following video clip.
In general, cisplatin appears to inhibit repair in mutant cells, leading to cell death; the difficult question to be answered is how do Pt 1,2-intrastrand cross-links inhibit repair?
The current hypothesis is that the binding of HMGB1 – one of a group of proteins known as high mobility group (HMG) proteins – with the 1,2-intrastrand cisplatin–DNA complex shields the DNA from intracellular repair leading to apoptosis. This binding occurs by means of HMG inserting a phenyl group protruding from its backbone into the notch created when cisplatin forms a complex with DNA. This also increases the bend in the DNA even more to about 60–90°, which facilitates the binding of a signalling protein called P53 which triggers a cascade of events leading to cell death. (However there is equally compelling evidence that suggests HMGB1 can cause these changes even in the absence of cisplatin-induced lesions so it is clear that these complex mechanisms still remain an area where much research is required.) Figure 9.31 shows schematically how these mechanisms are thought to operate.
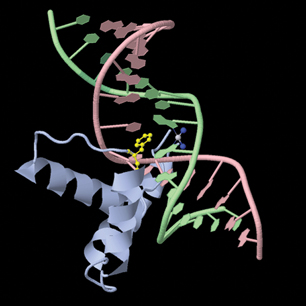
Figure 9.31 An HMG-domain protein (HMGB1; domain A shown as grey ribbon) inserts a phenyl group (yellow) into the groove created when cisplatin forms a complex with DNA, causing it to bend. A mutant protein lacking this phenyl group does not form a complex with cisplatin and DNA, suggesting that the phenyl group is crucial for complex formation. (Based on the pdb file 1ckt (Ohndorf et al., 1999).) Jmol version
As we saw previously (Section 9.11.6), transplatin is not active; the distance between the two Cl leaving groups is longer than in cisplatin, Figure 9.32, resulting in transplatin being unable to form 1,2-intrastrand DNA adducts similar to cisplatin. It can, however, form 1,3-intrastrand (G–p–N–p–G), 1,4-intrastrand and interstrand links as well as monoadducts.
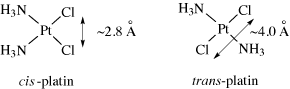
Figure 9.32 Distance between Cl atoms in cisplatin and transplatin.
The lack of anticancer activity for transplatin is believed to be because transplatin lesions are more easily repaired than those for cisplatin, as they lead to more radical distortion of DNA. They don’t bind HMG proteins as strongly as cisplatin lesions, possibly owing to the lack of an appropriate space for intercalation of the HMG protein phenyl group. In addition transplatin is more readily intercepted by sulfur-containing species (e.g. glutathione, GSH, 9.25) leading to removal of Pt from the cancer cells.

Cl- > NH3 in the trans effect so cisplatin is less susceptible to this reaction.
Transplatin monoadducts are more easily displaced by the action of trans-labilising nucleophiles such as glutathione or thiourea.
Once cisplatin has been introduced into the body, it will circulate in the bloodstream and so potentially can come into contact with all the organs of the body. The aim of cancer chemotherapy is to maximise damage to cancer cells whilst minimising damage to normal cells. As you will see, this is not totally achieved in practice, but essentially it is hoped that any excess cisplatin can be excreted from the body without too much harm to other cells. As a transition metal, platinum, by its nature, is able to bind to ligands available in the environment it finds itself in. As a soft metal, it will favour soft ligands and foremost amongst these in natural systems are sulfur atoms. Many biomolecules contain sulfur centres so these are clearly favourable potential coordination sites for platinum. Some of these binding interactions will reduce the efficiency of the drug and some of them will lead to undesirable side effects.
Intracellular thiols include glutathione, GSH (9.25). GSH is present in all cells, typically in concentrations of 3–10 mmol dm-3. It binds to Pt through sulfur to give a high molecular mass polymer with a Pt:GSH ratio of 1:2. GSH–Pt binding results in depletion of Pt from circulation and the Pt–GSH complex is pumped out from tumour cells. Clearly this affects the cytotoxic effect of the drug as it is removing the cisplatin before it can damage the cancer cell.
In the kidney, metallothionein (MT; Chapter 5) reacts with cisplatin to give Pt7-10MT containing PtS4 units, which are also inactive.
Cisplatin is a potent cytotoxic drug, which has limited targeting within the organism. By its nature, it will bind to DNA within cells regardless of whether those cells are cancerous or not. Fortunately there are aspects of the biochemistry of cancer cells which render them more susceptible to damage from cisplatin, but there are still serious side effects associated with both DNA- and protein-bound platinum (similar to other heavy metal poisoning (see Section 9.3)). This limits the maximum dose of cisplatin to about 100 mg per day for up to five consecutive days.
The toxic side effects can be partially controlled by inhibiting the formation of Pt–protein complexes or by ‘rescuing’ Pt from these Pt–protein complexes.
A high Cl- concentration in the solution containing the drug helps to inhibit the formation of RS-, RSH complexes, and hence cisplatin is administered in a saline drip. This reduces kidney damage dramatically.
Rescue agents such as 9.26 and 9.27:
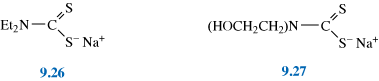
can be administered 3–4 hours after cisplatin treatment. These agents displace Pt from sulfur-containing biomolecules but do not affect Pt–DNA complexes. A typical treatment regime will involve infusions of cisplatin followed by infusions of rescue agents, followed by a period of rest to allow normal cells which have been damaged by the cytotoxic drug to recover. This cycle will typically be repeated several times to maximise the benefit of the treatment.
Some tumours have natural resistance to cisplatin whilst others develop resistance after initial treatment. Resistance arises through various mechanisms. First amongst these is the ability of the cancer to learn to recognise the lesions caused by cisplatin in DNA and to develop repair mechanisms to deal with them. However other processes also occur. Cancer cell membranes are generally ‘leaky’, which is a favourable adaptation to maximise the sequestration of nutrients. Generally therefore they are unselective in what they let into the cell. However over time they can develop protective mechanisms actively to pump the cisplatin back out of the cell and it will then be increasingly intercepted by sulfur-containing compounds. Finally, cancer cells are constantly mutating and therefore there is a good probability that by chance a mutation will arise that is resistant to cisplatin.
There are various approaches to these problems that include developing new Pt drugs (see next section); using a higher dosage; using combination chemotherapy with other active antitumour drugs (e.g. Taxol®); or using cisplatin in combination with other pharmacological agents. Much current cancer research is focused on looking at the biochemical mechanisms that make cancer cells successful and it is likely that metallopharmaceuticals will find applications in these areas in future.
Initially developments in platinum drugs tended to follow the structure–activity relationships outlined below.
In 1973 Cleare and Hoeschele proposed a loose set of ‘rules’ for platinum complexes to show those which might be expected to exhibit anticancer activity (Cleare and Hoeschele, 1973). They proposed that for a platinum complex of the general type PtL2X2 to show anticancer activity it must:
The anticancer activity is usually tested first in vitro on mouse leukaemia cell lines before testing on animals (Figure 9.33) and then there are several stages of clinical trials. The whole process can take many years. It has recently been estimated that only 1 in 10 000 screened drugs actually makes it to final approved clinical use.
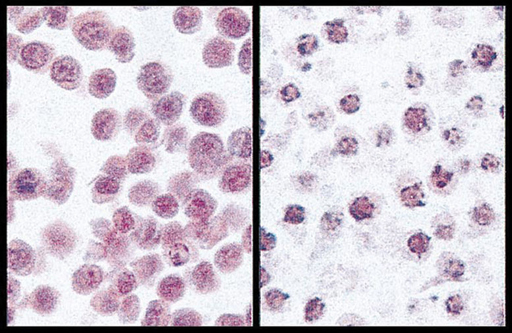
Figure 9.33 Histological analyses of acute myeloid leukaemia cells. The panels correspond to (a) untreated viable cells and (b) cells treated with cisplatin for 24 h (apoptotic). Source reference – (Czarnota et al., 1999).
Carboplatin, 9.28, was the first of the second generation Pt anticancer drugs to be approved for worldwide clinical use (since 1987 in the UK).
How does carboplatin follow the structure-activity rules above?
Carboplatin has two cis leaving groups, it is a Pt(II) complex, the leaving groups are likely to be moderately strongly bound as they form a chelate ring, it has two amine groups.
End of answer
Carboplatin is less toxic than cisplatin and this means that higher doses can be used (up to 2000 mg per day) and outpatient treatment is possible. The lower toxicity is mainly due to the added stability of carboplatin in the bloodstream, which prevents proteins from binding to it. This in turn reduces the amount of protein–carboplatin complexes to be excreted. The lower excretion rate of carboplatin means that more is retained in the body, and hence its effects are longer lasting. (A typical retention half-life for carboplatin is 30 hours, compared to 1.5–3.6 hours for cisplatin.)
However, carboplatin is less potent than cisplatin and approximately 4 times the dose is required for similar effectiveness. In addition, it is approximately 10 times as expensive.
As with cisplatin, carboplatin is administered via intravenous infusion and is active against the same range of tumours as cisplatin, forming similar adducts with DNA.
Nedaplatin, 9.29, the next drug to be approved, has similar clinical properties to carboplatin, and is predominately used in Japan for treatment of testicular, ovarian and cervical cancers.
Oxaliplatin, 9.30, was recently approved for clinical use (in 2002) under its trade name Eloxatin®. It is used in combination for the treatment of stage 3 colon cancers after surgery. This is an example of a drug with a 1,2–diaminocyclohexane ligand, often active against cisplatin resistant cancers.
In the next section we will consider the third generation Pt anticancer drugs which, as we will see, have moved on from the original structure–reactivity relationships proposed in 1973. But first, we end this section by returning to the final clip from the cisplatin story.
The next generation of anticancer drugs (many of which are currently undergoing clinical trials) have decreased toxicity, increased activity against cisplatin-resistant cells and/or the ability to be administered orally rather than intravenously.
This has been achieved by the design of a range of new platinum coordination compounds (as described below) with decreased reactivity towards nucleophiles, better biodistribution properties, different bonding modes to DNA and/or increased water solubility and stability.
Steric hindrance leads to decreased substitution reaction rates because of slower attack from nucleophiles. Crucially, slower reaction rates with sulfur-containing biomolecules leads to fewer side effects and can potentially overcome one type of resistance.

ZD0473, 9.31, is currently undergoing clinical trials for lung and ovarian cancers. Phase 2 results (2008/9) suggest this drug may be useful for patients with Pt-resistant ovarian cancer. The 2-methyl group sits above the plane of the molecule leading to axial steric hindrance. ZD0473 has been observed to form 1,2-intrastrand links with DNA similar to those formed by cisplatin.
The next series of drugs are complexes of Pt(IV) rather than Pt(II).
Why would you expect Pt(IV) centres to be more inert to ligand substitution than Pt(II) centres?
Pt(IV) complexes are six-coordinate and therefore more sterically hindered from attack. They are also 18-electron complexes and so have fewer vacant orbitals with which to form intermediates.
End of answer
Therefore Pt(IV) compounds are less likely to be intercepted by biomolecules and have potentially fewer side effects and are also active against some cisplatin-resistant cells. Although some studies have shown that these complexes can form Pt(IV) adducts with DNA resulting in a large kink in the DNA, it is perhaps more likely that they are slowly reduced to Pt(II) complexes by extracellular and intracellular agents and form the same adducts with DNA as shown by cisplatin.
Satraplatin, tetraplatin and iproplatin, 9.32–9.34, are all currently (2009) undergoing clinical trials. Satraplatin is an orally administered compound, which is soluble in water and in lipids; the ethanoate groups allow passage through the stomach lining. It is on trial for prostate cancer as well as some ovarian and lung cancers.

Drugs are also under development that combine the Pt complex with a second DNA binding function. By attaching DNA-intercalators such as doxorubicin or anilinoacridine (9.35, 9.36) to the active complex 9.37, it is envisaged that these compounds will localise on the DNA even before aquation of the platinum centre occurs.
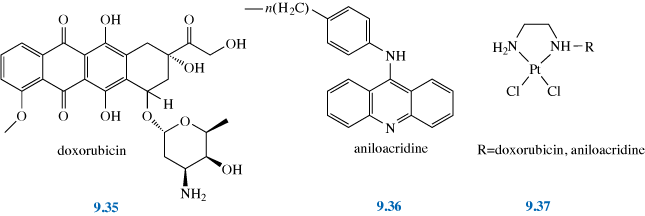
What advantage do you see that this might give to these drugs?
By adding a DNA binding function that can bind to the DNA before aquation you are reducing the delay before the drug become active.
End of answer
In addition, these binding functions can stabilise the DNA adducts after Pt binding at guanine. As these intercalators are potent anticancer drugs in their own right, the combination of heavy metal cytotoxity with traditional antimetabolite activity should be a powerful weapon particularly against cancers which are resistant to one or other of the agents acting alone.
Another approach to achieve the same effect is the addition of hydrogen-bonding groups to the complex, 9.38, which will bind to the DNA phosphate backbone; similarly, attachment of proteins or other molecules that are known to bind in the minor groove of B-DNA.

Sterically hindered ligands (as in 9.39 and 9.40) are used to reduce the kinetic activity of trans-platinum complexes resulting in aquation rates approaching those of cisplatin. This overcomes many of the problems encountered with transplatin being too easily intercepted by other biomolecules (see Section 9.11.9).
It is envisaged that the different adducts formed by trans-Pt complexes compared to cis ones will result in activity against cells which show resistance.
More recently, the search for anticancer drugs has led to the exploration of di- and tri-nuclear Pt complexes. Initially dinuclear Pt compounds,
e.g. [ClPt(NH3)2NH2(CH2)nNH2Pt(NH3)2Cl]2+, were studied and found to be active; they were able to chelate two guanines at N7 forming a hairpin structure with B-DNA. However these compounds turned out to be too toxic for clinical trials.
A trinuclear compound, BBR3464, 9.41, is now (2009) in clinical trials for melanoma, lung cancer and pancreatic cancers. It is very active against cisplatin-resistant cell lines and in addition is 10 × more potent than cisplatin. BBR3464 forms long-range interstrand and intrastrand cross-links with B-DNA (up to six base pairs apart) leading to significant unwinding of the duplex.

Complexes containing metals other than Pt have also been investigated for their anticancer properties. Perhaps the most obvious metals for study are the Group 10 analogues (Ni and Pd) of the active Pt(II) complexes. However, these complexes undergo fast ligand substitution and so they are unsuitable for clinical use. Au(III) is isoelectronic with Pt(II) and the use of Au(III) complexes has been explored for possible anticancer activity although the Au(III) analogue of cisplatin is again too reactive. The reduction of Au(III) to Au(I) is also a problem, although this can be controlled by careful choice of ligands such as the orthometallated benzylamine ligand (DAMP) in 9.42, [Au(DAMP)X2] where X = Cl-, O2CCH3-. This complex shows similar activity to cisplatin for certain cancers, however as its mode of action is different it may be of potential use against cisplatin resistant tumours.

Transition metal complexes containing cis chloride ligands have also been investigated. Titanocene dichloride [(C5H5)2TiCl2] showed promise against lung and breast cancer in mice and underwent clinical trials for cancer treatment in the 1990s although these proved unsuccessful and have now been abandoned. It was originally thought that [(C5H5)2TiCl2] could form adducts with DNA in a similar manner to cisplatin due to cis metal–chloride bonds. However subsequent studies showed that it only binds weakly to DNA bases and more strongly to the phosphate backbone. Another of the problems encountered was that the cyclopentadienyl ligand can be readily displaced or protonated, for example on reaction with transferrin in the blood.
Ruthenium compounds are currently of much interest as anticancer drugs because their reaction kinetics are similar to those of Pt(II). Ruthenium is also well suited for pharmaceutical applications and its compounds have been studied as immunosuppressants, antimicrobial agents and antimalarials. [RuCl3(NH3)3] was originally shown to exhibit anticancer activity although it proved too insoluble for clinical use.
The Ru(III) complexes NAMI-A, 9.43, and KP1019, 9.44, are currently both undergoing clinical trials. KP1019 is cytotoxic (kills cells) whereas NAMI-A shows antimetastatic activity (it prevents the spread of cancer).

These complexes bind to guanine N7, and also to A and C bases. However, they both have octahedral structures, and the bulky axial substituents prevent them from forming the same structures with DNA as cisplatin. Ru(III) is relatively inert towards ligand substitution, but it is possible that it undergoes in vivo reduction to give the more labile Ru(II) complexes. Ru(III) may also interfere with protein binding, and uptake of iron by cancer cells.
Cancer cells have a high number of transferrin receptors on their surface, requiring iron for cell division. How might this be effected by Ru(III)?
Ru(III) is a hard ligand and binds with transferrin, blocking the uptake of iron by the cells.
End of answer
Another metal that is also taken up by transferrin is Ga(III). The complex [Ga(III) (maltolate)3], 9.45 has shown promise as a possible anticancer drug, with rapid uptake and almost complete transfer of Ga(III) on to transferrin. A related drug entered clinical trials in 2003.
Sn and As compounds have been found to exhibit activity against certain types of leuk a emia. For example, As 2 O 3 is used to treat acute promyelocytic leuk a emia, with very successful remission rates. Arsenic is of course generally toxic, however in the small quantities used in treatment it is selective for killing particular types of cancer cell. At physiological pH , As 2 O 3 undergoes hydrolysis to As(OH) 3 . This is metabolised, undergoing oxidative methylation to As(V) followed by reduction to produ ce mono–, di– and tri– methylated As(III) species. These methylated organic As(III) species have greater potency as cytotoxins. These species are believed to target thiols in key enzymes and proteins, in particular those involved in the main redox pathways in mitochondria. This results in increased production of reactive oxygen species, leading to apoptosis.
In Section 9.9 we examined the use of radionuclides in diagnostics, but radionuclides also have a role in therapy. Radiotherapy is the use of high doses of radiation for the destruction of cancerous tissue, for example 131I is used in the treatment of thyroid cancer. External sources of radiation suffer from problems in controlling the dose given to the tumour and also because of exposure of healthy tissue to the radiation. In theory, internal irradiation from radioisotopes should be more controllable. The radioisotope can be used to deliver radiation selectively and intensively to a tumour. The radionuclide is incorporated into a chemical complex that can target only the diseased tissue. Most of the radioisotopes used are ß--emitters, although some a-emitters have been proposed.
For radiotherapy agents to be effective, they must selectively reside in the tumour and stay there for a sufficient time to be able to deliver a high enough dose to destroy cancerous tissue. They must also clear the kidneys quickly to protect these and other organs from irradiation. In addition the radionuclide must be prepared with high purity. Some examples of radionuclides used for radiotherapy are shown in Table 9.7, together with some of their properties.
Table 9.7 Properties of the radioactive isotopes (ß--emitters) used for radiotherapy.
| Half-life | Max ß- energy/MeV | Average (max) range in tissue/mm | ? energy/keV | Use | |
|---|---|---|---|---|---|
| 186Re | 91 h | 1.08 (71%) | 0.33 (5) | 137 (9%) | bone cancer |
| 188Re | 17 h | 2.21 (85%) | 0.64 (11) | 155 (15%) | bone cancer |
| 90Y | 65 h | 2.3 (100%) | (12) | bone and liver cancer | |
| 89Sr | 50 days | 1.46 (99%) | 2.4 (8) | bone cancer | |
| 153Sm | 46 h | 0.68 (32%) | 0.55 (3.4) | 103 (29%) | bone cancer |
| 131I | 8.02 days | 0.606 (90%) | 364 (81%) | thyroid cancer |
The following video clip shows an example of the application of radionuclide; in this example radioactive iodine to treat thyroid cancer. Although iodine is not a metal and so is strictly outside the scope of this chapter, this clip provides a good overview of this type of ‘unsealed source’ radiotherapy using ß--irradiation.
There are two rhenium and one yttrium isotopes which are particularly suitable for therapeutic use by means of ß--irradiation. We will study these in the next section.
Which rhenium isotope is more suitable for irradiating larger tumours?
188Re is more appropriate for larger masses as the ß--particles emitted have higher energy and hence a deeper penetration depth.
End of answer
We previously met the two rhenium isotopes, 186Re and 188Re. 188Re is easier and cheaper to prepare than 186Re, although its short half-life can be problematic. It is prepared from the radioactive decay of 188W using similar ion-exchange methods to those used for 99mTc. 186Re can be prepared from neutron irradiation of 185Re in a reactor; the yield is only low to medium and contamination with non-radioactive 185Re leads to reduced specific activity.
90Y is a high-energy pure ß--emitter (2.3 MeV, 100%) with a half-life of 65 h. The maximum range of its emitted ß--particle in tissue is 12 mm. 90Y is produced in a generator from the decay of 90Sr. The half-life of 2.7 days (65 hours) is long enough to allow the radiopharmaceutical to be manufactured and delivered for clinical use but short enough to achieve a critical dose rate.
Rhenium is a Group 7, third transition series metal and therefore lies directly below Tc in the Periodic Table. Owing to the lanthanide contraction, the complexes of Tc and Re are very similar in physical characteristics – size, lipophilicity, etc. However Re complexes are easier to oxidise, harder to reduce and more kinetically inert. Re complexes can often oxidise in vivo to give [ReO4]- and this allows excess Re to be more readily removed via the kidneys.
Yttrium, coming under scandium in the Periodic Table, is at the beginning of the second transition series in Group 3. It is often referred to as a ‘pseudo-lanthanide’ owing to its similar properties (charge and ionic radius) to the lanthanides. Yttrium favours the +3 oxidation state and forms predominantly ionic bonds. Coordination numbers vary between 7 and 10, but stable complexes with polydentate ligands with eight or nine donor atoms, for example (dota)4- (Figure 9.9), are common.
External radiation sources are widely used for the relief of localised bone pain such as a single primary tumour. However, when a cancer has metastasised (i.e. the cancer has spread and formed secondary tumours) and growths are widely distributed through the skeletal structure, radiopharmaceuticals have proved to be more effective.
Therapeutic rhenium radiopharmaceuticals for treatment of bone pain have been developed using analogous coordination chemistry to that used for the 99mTc-based bone-imaging agents (see Section 9.9.6). The diphosphonate groups are able to bond to the Ca2+ ions in bone.
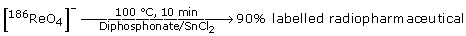
Other radionuclides that have been used in the palliation of bone pain include 89Sr and 153Sm. 89Sr is administered as an ionic chloride salt (89SrCl2).
Why would radioactive strontium be an obvious choice for bone treatment?
The coordination chemistry of strontium is very similar to that of calcium (both are in Group 2) and it is therefore able to replace calcium in the bones. It has been observed to concentrate 5 to 10 times more in metastases than in healthy bone.
End of answer
What other approach could be used for targeting bone tissue besides a calcium substitute?
Using a ligand that bonds to calcium.
End of answer
153Sm can be administered as a 153Sm–EDTMP complex. The phosphonate groups on the EDTMP (9.46) ligand have a high affinity for the Ca2+ cations in bone.
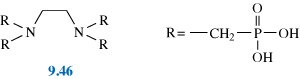
As well as its use in imaging, iodine, as the 131I isotope (administered as NaI), can be used to treat various types of thyroid carcinomas as we saw in the video clip in section 9.14. As 131I emits ?-rays as well as ß--particles (Table 9.5), it is possible to monitor accurately, and therefore control, the radiation dosage administered. However, there are still some questions concerning the impact of 131I on non-diseased parts of the body.
An alternative drug used to treat thyroid cancer is a Re(V) square-pyramidal complex with an apical oxo group and two DMSA ligands, 9.47. (A Tc(V) complex containing the same ligands has been used for imaging to monitor the progress of this disease.)

Steroids can be attached to Re-chelated metal centres in order to target tissue rich in particular steroid receptors. The Re metal centre is usually held inside a N2S2 or similar chelate system, analogous to that previously discussed for Tc metal centres (see Section 9.9.7). However alternative approaches have also been investigated, such as 9.48.

Certain tumours (pituitary, malignant breast, pancreatic) have a large number of receptors for somatostatin (a tetradecapeptide) and its cyclic analogue, octreotide. Octreotide has disulfide bridges and reacts with ReO4- and stannous citrate to give the Re-labelled peptide presumably via reduction of the disulfide bridges.

90Y DOTA-octreotide, 9.49, has also been investigated as a therapeutic agent.
Radiolabelled antibodies are often referred to as ‘magic bullets’ due to their high specificity for and lethal effect on the targeted tissue. To date the main clinical application of radioimmunotherapy (RIT) is for the treatment of non-Hodgkin’s B-cell lymphoma using 90Y (or 131I) labelled monoclonal antibodies (MAbs; see Section 9.9.7). However, treatment of solid tumours using RIT has proved difficult due to the low uptake of the MAb fragments into the tumours. Low uptake leads to damage to other body tissues from unbound MAb-radioisotope complexes. One potential way to avoid this is to engineer the MAbs biochemically to make them more humanised (i.e. less mouse-like).
Pre-targeting techniques are also under investigation to circumnavigate this problem. Pre-targeting uses a pair of mutually high affinity molecules, e.g. avidin/biotin. The technique here is first to administer avidin-tagged MAb and wait about 24 h for maximum tumour binding. The remaining MAb is then removed from the bloodstream using biotin-tagged human serum albumin (which is cleared via the liver). Next the radioisotope is added in a small biotin-containing molecule (e.g., DOTA-biotin) which can bind to the avidin and thus the tumour. The unbound radioisotope then clears quickly due to its low molecular mass.
In this, and the following sections, we aim to provide you with a brief introduction to some of the other uses of metals in therapy. These are supplemented with suggested reading material that will provide you with further background.
Metal compounds are used as chemotherapeutic agents in medical applications other than cancer therapy. The application of chemotherapeutic agents results in cell-death. An early example was the use of arsphenamine (salvarsan) to treat syphilis. Metal containing chemotherapeutic agents are currently in use as antiparasitic agents for the treatment of tropical disease and also for antibacterial (or antibiotic) applications. We will consider these briefly below.
Tropical diseases such as malaria, leishmaniasis and trypanosomiasis are examples of parasitic diseases and constitute a major world health problem with the potential to affect millions of people across Africa, Asia and South America in particular. A number of these are currently treated with metal containing chemotherapeutic drugs. These drugs were first introduced over 50 years ago and in some areas of the world parasites are developing resistance to the drugs. Development of new drugs for tropical diseases has been very limited. This has led to the development of the ‘Drugs for neglected diseases initiative’(DNDi).
an independent non-profit making organisation involving Médecins Sans Frontières and other partner organisations.
The following video produced by Médecins Sans Frontières looks at the need for further research in the field of neglected diseases.
One example where metal complexes are currently employed is the use of organic pentavalent antimony compounds in the treatment of leishmaniasis, a disease caused by parasites transmitted by certain species of sand fly. Two major forms of leishmaniasis which are particularly problematic are: cutaneous leishmaniasis whose main symptoms include sores on the skin; and the more severe form, visceral leishmaniasis, or Kala azar, which causes damage to the spleen and liver, and if left untreated is fatal. Currently over 50 000 people die every year from leishmaniasis. More information on this disease can be found by following the suggested links in Further Reading.
The two main antimony chemotherapeutic agents licensed for use are sodium stibogluconate (Pentostam®) and meglumine antimoniate (Glucantime®) which are administered via intravenous or intramuscular injection. In use since the 1940s, resistance to these drugs is evident in certain parts of the world. In addition, the treatment is both long and painful.
Both drugs contain pentavalent antimony, however it is believed that the antimony is reduced to Sb(III) in vivo, although the mechanisms by which this occurs and how the drug functions are unclear. The antimony may target the white blood cells (macrophages) destroying the parasite.
The following video produced by Médecins Sans Frontières, considers the treatment of Kala azar.
Another disease which has typically been treated with a metal-containing drug is trypanosomiasis or human African sleeping sickness. The parasite responsible is spread by the tsetse fly and is particularly widespread in Sub-Saharan Africa. The disease kills tens of thousands each year, affecting the central nervous system, causing convulsions and serious sleep disturbance, leading to coma and death. Melarsoprol, the main drug used in the treatment of sleeping sickness, contains arsenic and itself kills around 5% of patients to whom it is given. Again treatment is long and painful and the effectiveness of the drug appears to have diminished having been in use for 60 years. As in the case of the drugs for leishmaniasis, the mode of action for this drug is not certain. Some alternatives are available, the latest campaign using a combination of two non-metal containing drugs (nifurtimox-eflornithine combination therapy or NECT) was launched in 2009.
The following video, produced by Médecins Sans Frontières, looks at the current and alternative treatments for sleeping sickness.
This was also discussed in a recent edition (January 2010) of the Lancet, ‘Killer coma: the evolving story of sleeping sickness treatment’ (The Lancet, Volume 375, Issue 9709, Page 93, 9 January 2010)
As well as research into organic alternatives for the treatment of tropical disease, research for metal-based chemotherapeutic agents is also ongoing. The reviews suggested in Further Reading highlight some of the ongoing work in this field.
Metals are also used in medicine for their antibacterial properties. The antibacterial effect of silver, for example, has been exploited throughout history, with early examples dating back to the treatment of ulcers with silver nitrate solution. More recently silver has been used in the treatment of wounds, especially burns. The active form of silver is thought to be silver(I) which is released slowly into the wound. Silver is also used in bandages and as a coating for medical devices such as catheters and is even used for non-medical uses such as in washing machines.
One such agent is silver sulfadiazine which combines silver nitrate with an organic antibacterial agent sulfadiazine. This is typically applied as an ointment. Wound dressings containing silver in a number of different forms including nanocrystalline particles of silver, are also used clinically. In all cases the key aim is to provide sustained release of Ag(I) at the required concentration. The Ag+ ions released are believed to bind to and inhibit respiratory enzymes in the microbial electron transport chain.
More detailed information on the use of silver as an antimicrobial agent can be found in the reviews suggested in Further Reading.
As discussed previously (Section 9.10) metals have been used for a diverse range of therapeutic medical applications other than those exploiting their ability to kill cells. In this section we will briefly consider some of the examples of metal drugs that are used specifically to target and modulate cellular responses. An example is the use of gold drugs for the treatment of arthritis. Another is the ability to control levels of NO. We will also consider the use of metal complexes as mimics for natural processes involving metal ions. Examples include manganese superoxide dismutase mimics and also vanadium compounds which are believed to act as insulin mimics, enhancing the natural action of insulin.
Gold has been valued for centuries, as far back as the time of the ancient Egyptians, not only as a precious metal, but also for its healing powers. In more recent times, gold complexes have been found to help people with rheumatoid arthritis, a debilitating and painful inflammatory condition where the cartilage between bone joints is lost over time. The administration of gold-containing drugs is often referred to as chrysotherapy, named after Chryseis, the golden-haired nymph of Greek mythology. Gold compounds were first used to treat rheumatoid arthritis in 1929 when a French physician, Jacques Forestier, investigated the use of Au(I) thiolates, used around that time to treat tuberculosis, an ailment he believed to be related to arthritis.
Rheumatoid arthritis is caused by the body’s immune system not operating properly so that it turns on itself causing inflammation (an autoimmune disease). It is thought that the gold drugs interact with the immune system of the body helping to stop this process. They appear to block the release of a particular molecule (HMGB1) which stimulates the immune system and which is particularly prevalent in the synovial tissue and fluid around bone joints in sufferers of this disease..
A number of gold drugs have been developed for the treatment of arthritis. The earliest ones were injected directly into the inflamed joints, with understandable patient refusals. Much research has gone into developing gold drugs that can be taken orally or can be injected into the bloodstream. The most recent of the gold drugs to be used is auranofin, see structure 9.50, which is taken orally.

Read Section 4.43, pages 285–291 of the RSC eBook Medical Applications of Coordination Chemistry (Jones and Thornback, 2007). The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.11.
You should also watch the following video clip which highlights related research that has suggested a link between wearing gold rings and arthritis prevention.
The gold drugs discussed in the article typically contain Au(I) and sulfur ligands (other than aurofin which also contains a phosphine ligand).
Why do the Au(I) drugs contain S (and P)?
Au(I) is a soft acid and so will tend to form the most stable complexes with S and P ligands (soft bases).
End of answer
The chemistry surrounding how these drugs operate is not yet well understood, however the drugs essentially provide a source of biologically available gold.
What is the main role of the ligands in these complexes?
The role of the ligands appears to be just to confer the necessary absorption and transport properties to the pharmaceutical agent; ligand exchange occurs rapidly in gold compounds. In auranofin, the ester groups confer sufficient aqueous solubility and the triethyl phosphine the lipophilicity for membrane transport. Once in circulation in the body, the ligands are substituted by the protein albumin in the blood serum, coordinating through the sulfur atom of cysteine residues. Research focuses on finding compounds that will remain localised in the joints.
End of answer
What possible functions have been proposed for the anti-inflammatory effect of Au(I)?
Au+ may bind to cysteine thiolate groups at DNA binding sites.
End of answer
Long-term gold therapy does have a variety of side effects, some associated with metabolites of the drug, including higher oxidation states of gold, such as Au(III), which may cause cancerous changes. Au(0) may be deposited in the cornea and lens of the eye and ocular changes are observed in as many as 95% of long-term treated patients.
NO plays a number of important roles in the body, one of which is to regulate dilation of the blood vessels in the cardiovascular system. Overproduction of NO, for example due to infection can be a problem. It is also sometimes necessary to use drugs to release NO to improve vasodilation and alleviate severe high blood pressure (hypertension). Both can be treated using metal complexes, as outlined in Activity 9.12 below.
Read Section 4.6.3, pages 306–309 of the RSC eBook Medical Applications of Coordination Chemistry (Jones and Thornback, 2007). The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.12.
As you read this article consider the problems with the drug used clinically to treat acute hypertension, Na2[Fe(CN)5(NO)].2H2O (Nitropress®) and the Ru complexes currently under investigation for this purpose. Consider also the desirable properties for NO release and how by choosing certain ligands the properties of the complex can be manipulated to either act as a NO scavenger or to release NO.
Bipolar affective disorders such as manic depression are commonly treated with lithium, typically administered orally as Li2CO3 tablets. After administration the lithium is distributed in the bloodstream and tissues as Li(I). Li+ is a small, labile ion that is readily transported across cell membranes.
The exact role of the Li(I) is unclear, however amongst a number of possibilities it is believed to inhibit an enzyme responsible for production of the molecule inositol. Overproduction is believed to cause an imbalance in the neurotransmitters in patients with bipolar disorders. Li(I) binds to one of the two Mg(II) sites in the active site of the enzyme, inhibiting action of the enzyme and hence production of inositol.
Insulin was discovered in 1922 and its structure famously elucidated in 1969 by the Nobel Prize winner, Dorothy Hodgkin. It is a signalling hormone, made in the pancreas, which controls the metabolism of fat and carbohydrate in the body and thus the supply of glucose. There are two types of diabetes: Type I ‘insulin dependent’ diabetes is the condition where the body cannot produce sufficient insulin; Type II non-insulin dependent or ‘insulin resistant’, accounting for about 90% of cases, usually appears in older people, and although insulin is produced it cannot be used. In both cases the glucose level in the blood increases and can be detected in the urine providing a characteristic test for diabetes. The discovery of insulin revolutionised the treatment of Type I diabetics; however, it cannot be taken orally and has to be injected subcutaneously. This method of treatment also works for many Type II patients, but alternative drugs that mimic the effect of insulin are also under research for Type II diabetes..
What features would be necessary for such a drug?
The drug should be readily absorbed, sufficiently stable that it can be distributed as required, non-toxic, with ideally no side effects and should demonstrate specificity to the action required, in this example mimicking the action of insulin and preferably should be able to be taken orally.
End of answer
As early as 1899 it was observed that diabetic patients excreted less glucose in their urine when treated with sodium vanadate (Lyonett and Martin, 1899). However with the subsequent discovery of insulin research moved away from insulin substitutes and it was not until the 1980s, when it was found that orthovanadate solutions enabled the uptake of glucose in experimental diabetic rats, that intensive research into finding effective and safe vanadium-containing insulin mimics became active. Orthovanadate, VO43-, a species somewhat akin to phosphate, exists in solution as HVO42- and H2VO4-, again similar to the hydrogen phosphates, although at pH 7.2, as under physiological conditions, it is predominantly H2VO4-. Subsequent studies showed that vanadyl, VO2+, salts are also active; vanadyl has some similarities to Mg2+.
What are the oxidation states of vanadium in these species?
VO43-, HVO42- and H2VO4- all contain V(V); VO2+contains V(IV).
End of answer
Research has concentrated on developing vanadium salts with organic ligands that improve the solubility and transport properties of the mimics as well as reducing toxicity. The mechanism of action of both insulin and vanadate is very complex, but vanadate has been shown to inhibit the action of some of the enzymes in the liver which act to store glucose and also to block other hormones which stop insulin from working.
Some success has been achieved with oxovanadium(IV) compounds chelated by maltolato ligands, 9.51:
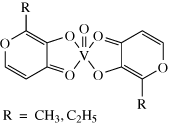
Two such complexes, BMOV and BEOV, containing the maltol and ethyl maltol ligands respectively, show much better activity than vanadyl sulfate, VOSO4, and also have fewer side effects. They appear to increase the permeation into tissues via passive diffusion. BEOV completed Phase I clinical trials in 2000. A small Phase IIa trial reported positive results in 2008. The extract in Activity 9.13 explores vanadium ‘insulin mimics’ in more detail.
You should now read Medical Applications of Coordination Chemistry (Jones and Thornbank, 2007) Sections 4.5.1–4.5.5, pages 292–298, and then answer the question below. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.13.
What are the key roles for the ligand?
The active species is believed to be the vanadyl ion (formed on dissociation of the complex), with the ligand providing sufficient stability to the complex to allow absorption but not so much that the complex fails to dissociate when in the bloodstream.
The vanadyl is believed to be distributed around the body by transferrin, or serum albumin, in the blood. It is thought to affect insulin signalling and enable greater regulation of glucose metabolism and homeostatis. It is believed to do this by inhibiting one of the enzymes involved in insulin regulation; this leads to an increase in insulin activity and hence greater regulation of glucose metabolism.
End of answer
Vanadium compounds are not used to treat Type I diabetes and are only effective if insulin is present such as in Type II diabetes; as such they strictly enhance rather than mimic insulin.
We have seen throughout this book that metals are essential for many physiological processes. The next area we will consider is the role of metal drugs as mimics of natural processes. One particular example is the development of superoxide dismutase mimics.
What is the role of superoxide dismutase (SOD) in the body?
SOD removes the damaging superoxide ions, O2-, produced in respiration by catalysing the conversion (dismutation) of O2- into the less harmful O2 and H2O2.
End of answer
Occasionally, for example as a result of disease or trauma, production of superoxide is too high to be effectively controlled by SOD. In such cases pharmaceutical SOD mimics could be used to remove the damaging superoxide ions.
From your knowledge of SOD which metals are likely to be useful in the design of SOD mimics?
The two examples discussed in Chapter 8 (Section 8.2.6) were Cu–Zn SOD and Mn SOD. Both Cu and Mn possess variable oxidation states differing by one oxidation state and so are suitable for one electron transfer. Fe is another possibility, also possessing two oxidation states differing by one (indeed, Fe–SOD is found in bacteria such as E. coli).
End of answer
The metal that appears to show most promise pharmaceutically is Mn and a number of Mn SOD mimics are undergoing clinical trial. Activity 9.14 looks at this area of research in more detail.
You should now read Medical Applications of Coordination Chemistry (Jones and Thornback, 2007) Sections 4.6.2, pages 299–305, which can be found on the website in PDF format. As you read the article you should consider the choice of both Mn and the ligands used in these SOD mimics. The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following link: Extract for Activity 9.14.
As well as their use in treating metal overload, chelating ligands can also be used to target metals in biological processes. One such example is a group of zinc-containing enzymes known as matrix metalloproteinases (MMPs), which aid the breakdown of connective tissue such as collagen or blood vessels. They are important in processes such as wound healing, apoptosis and embryo development. Over expressions of these enzymes, in tumours for example, can have a detrimental effect, playing a role in a number of serious conditions including cancer, rheumatoid arthritis and diseases of the central nervous system. Naturally occurring inhibitors of these enzymes have been found to contain zinc-binding groups, together with one or more functional group that can form hydrogen bonds with the enzyme backbone. Research is underway to find synthetic inhibitors to mimic these natural inhibitors by chelating the Zn(II) in the MMPs.
Synthetic chelating ligands typically utilise hydroxamate, carbonate or thiolate groups. A number of synthetic inhibitors have been tested in clinical trials for the treatment of breast and ovarian cancers, however they generally show poor selectivity and in some cases have been found to have musculoskeletal side effects. Current research is centered on the design of more selective inhibitors for particular enzymes. Such chelating ligands have also been under research to target zinc-finger proteins, including those important for HIV replication.
We will now briefly consider a very different way that metals and metal ions can be used in medicine – the example of synthetic materials for bone and dental implants, in particular in hip and knee replacements and in dental amalgam.
The following activity introduces the use of synthetic materials in medicine and in particular the use of metal alloys such as cobalt–chromium, stainless steel or titanium (with 6% aluminium and 4% vanadium, referred to as Ti–6Al–4V) for hip and knee replacement surgery.
You should now read pages 1–9 and 107–109 of the RSC eBook Chemistry of Medical and Dental materials, by J.W. Nicholson (Nicholson, 2002), and then answer the questions below.The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following links: Extract a for Activity 9.15 and Extract b for Activity 9.15.
What properties are important when considering the use of metal alloys as biomaterials?
The alloy should be biocompatible, able to withstand the corrosive environment in the body, have a high strength and resist fatigue.
End of answer
How can osseointegration, that is the growth of bone right up to the implant, be encouraged?
By roughening the surface of the alloy or coating the surface of the implant with plasma-sprayed hydroxyapatite.
End of answer
One of the biomaterials under study for use as bone implants is hydroxyapatite.
What advantages are there to using this material for bone implants?
Hydroxyapatite is a natural component of bone and so will not be rejected by the body.
End of answer
Synthetic hydroxyapatite however tends to be less durable or porous than natural bone. To mimic bone, small particles of hydroxyapatite are required, containing calcium ion vacancies and carbonate ions in the lattice.
How does carbonate substitution on the phosphate lattice balance calcium ion vacancies?
Carbonate has a charge of -2 whereas phosphate has a charge of -3. Calcium ion vacancies will tend to make the overall crystal negatively charged; this can be offset by reducing the anion charge from -3 to -2.
End of answer
Standard chemical synthetic processes tend to produce materials with insufficient carbonate or with carbonate in the wrong position. Research is underway into biomimetic preparation routes, which involve implanting a starting material such as calcium phosphate and using the enzymes in the biological fluids in the body to produce new bone.
Metals are also used in dentistry, as implants or in amalgams. The following activity considers this in some detail.
You should now read pages 15–17 and 136–140 of the RSC eBook Chemistry of Medical and Dental Materials, (Nicholson, 2002), which can be found on the website in PDF format.The complete book can be accessed through the RSC eBook collection, however this extract is also available as a PDF file by clicking on the following links: Extract a for Activity 9.16 and Extract b for Activity 9.16.
Why are titanium alloys particularly suitable for use as dental implants?
Titanium alloys are biocompatible with bone and also have been shown to have the ability to osseointegrate.
End of answer
What factors effect the durability of dental amalgams?
The composition of the mixture used in the amalgam, how well it is mixed, control of moisture during the process, and preparation of the cavity are all important when applying the amalgam, as are oral health and hygiene as well as tooth grinding by the patient after application.
End of answer
The material covered in this chapter is designed to provide you with an insight into the fascinating field of Metals in Medicine. The chemistry covered is diverse, reflected in the range of applications covered. By necessity we have only provided an overview of this vast field of research, much of which is rapidly moving. Should you wish to further your studies in this fascinating area, a section on suggested further reading is provided at the end of this chapter.
Cleare, M.J. and Hoeschele, J.D. (1973) ‘Antitumor platinum compounds – relationship between structure and activity’, Platinum Metal Review, vol. 17, iss. 1, pp. 2-13. http://www.platinummetalsreview.com/pdf/pmr-v17-i1-002-013.pdf
Cohen, M.S. and Bookheimer, S.Y. (1994) ‘Localization of brain function using magnetic resonance imaging’, Trends in Neuroscience, vol. 17, iss. 7, pp. 268-277.
Czarnota, G.J., Kolios, M.C., Abraham, J., Portnoy, M., Ottensmeyer, F.P., Hunt, J.W. and Sherar, M.D. (1999) ‘Ultrasound imaging of apoptosis: high-resolution non-invasive monitoring of programmed cell death in vitro, in situ and in vivo’, British Journal of Cancer, vol. 81, no. 3, pp.520-27.
Duffus, J.H. and Worth, H.G.J. (eds.) (1996) Fundamental Toxicology for Chemists, Cambridge: RSC Publishing.
El Saghir, N.S., Elhajj, I.I., Geara, F.B. and Hourani, M.H. (2005) ‘Trauma-associated growth of suspected dormant micrometastasis’, BMC Cancer, vol. 5,
Gelasco, A. and Lippard, S.J. (1998) ‘NMR solution structure of a DNA dodecamer duplex containing a cis-diammineplatinum(II) d(GpG) intrastrand cross-link, the major adduct of the anticancer drug cisplatin’, Biochemistry, vol. 37, no. 26, pp.9230-39.
Huang, H., Zhu, L., Reid, B.R., Drobny, G.P. and Hopkins, P.B. (1995) ‘Solution structure of a cisplatin-induced DNA interstrand cross-link’, Science, vol. 270, pp.1842-45.
Jones, C.J. and Thornback, J.R. (2007) Medicinal Applications of Coordination Chemistry, Cambridge: RSC Publishing.
Itoh, K., Fujii, Y., Suzuki, K. and Nakada, T. (2001) ‘Asymmetry of parietal lobe activation during piano performance: a high field functional magnetic resonance imaging study’, Neuroscience Letters, vol. 309, iss. 1, pp. 41-44.
Lloyd, N.C., Morgan, H.W., Nicholson, B.K., Ronimus, R.S. (2005) ‘The composition of Ehrlich’s salvarsan: resolution of a century-old debate’, Angewandte Chemie International Edition, vol. 44, no. 6, pp. 941-944.
Morgan, N.F. and Nowotnik, D.P. (1999) ‘Development of dopamine transporter imaging agents for the diagnosis of Parkinson’s disease’, Drug News & Perspectives, vol. 12, no. 3, pp. 137
Nicholson, J.W. (2002) Chemistry of medical and dental materials, Cambridge: RSC Publishing.
Sou, Z., Lippard, S.J. and Johnson, K.A. (1999) ‘Single d(GpG)/cis-Diammineplatinum(II) Adduct-Induced Inhibition of DNA Polymerization’, Biochemistry, vol. 38, no. 2, pp. 715–726
Williams, D.R. and Taylor, D.M. (1995) Trace Elements Medicine and Chelation Therapy, Cambridge: RSC Publishing.
Allardyce, C.S. and Dyson, P.J. (2001) ‘Ruthenium in Medicine: Current Clinical Uses and Future Prospects’, Platinum Metals Review, vol. 45, no. 2, pp. 62-69.
Cohen, S.M. (2007) ‘New approaches for medicinal applications of bioinorganic chemistry’, Current Opinion in Chemical Biology, vol. 11, iss. 2, pp. 115-120.
Farrell, N.P. (ed.) (1999), Uses of Inorganic Chemistry in Medicine, Cambridge: RSC Publishing.
Guo, Z. and Sadler, P.J. (1999) ‘Metals in Medicine’, Angewandte Chemie International Edition, vol. 38, iss. 11, pp. 1512-1531.
Schatzschneider, U. and Metzler-Nolte, N. (2006) ‘New Principles in Medicinal Organometallic Chemistry’, Angewandte Chemie International Edition, vol.45, pp. 1504-1507.
Thompson, K.H. and Orvig, C. (2003) ‘Boon and bane of metal ions in medicine’, Science, vol. 300, no. 5621, pp. 936-939.
If you are interested, more information on essential minerals and their recommended intakes can be found at the following sites.
UK Food standards agency
http://www.eatwell.gov.uk/healthydiet/nutritionessentials/vitaminsandminerals/
US Department of Agriculture Food and Nutrition Information Centre –
Links to Tables of Dietary Reference Intakes (DRI)
http://fnic.nal.usda.gov/nal_display/index.php?info_center=4&tax_level=2&tax_subject=256&topic_id=1342
An interactive site that can be used to calculate personal dietary nutrient recommendations
http://fnic.nal.usda.gov/interactiveDRI/
World Health Organisation recommendations
http://whqlibdoc.who.int/publications/2004/9241546123_annexes.pdf
Anderson. O. (1999) ‘Principles and Recent Developments in Chelation Treatment of Metal Intoxification’, Chemical Reviews, vol. 99, iss. 9, pp. 2683-2710.
Liu, Z.D. and Hider, R.C. (2002) ‘Design of iron chelators with therapeutic application’, Coordination Chemistry Reviews, vol. 232, iss. 1-2, pp. 151-171.
Peralta-Videa, J.R., Lopez, M.L., Narayan, M., Saupe, G. and Gardea-Torresdey, J. (2009) ‘The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain’, The International Journal of Biochemistry and Cell Biology, vol. 41, iss. 8-9, pp. 1665-1677.
Sarkar, B. (1999) ‘Treatment of Wilson and Menkes Diseases’, Chemical Reviews, vol. 99, iss. 9, pp. 2535-44.
Blower, P. (2006) ‘Towards molecular imaging and treatment of disease with radionuclides: the role of inorganic chemistry’, Dalton Transactions, issue 14, pp. 1705-11.
Volkert, W.A. and Hoffman, T.J. (1999) ‘Therapeutic Radiopharmaceuticals’, Chemical Reviews, vol. 99, iss. 9, pp. 2269-92.
Hall, M.D. and Hambley, T.W. (2002) ‘Platinum(IV) antitumour compounds: their bioinorganic chemistry’, Coordination Chemistry Reviews, vol. 232, iss. 1-2 pp.49-67.
Ott, I. (2009) ‘On the medicinal chemistry of gold complexes as anticancer drugs’, Coordination Chemistry Reviews, vol. 253, iss. 11-12, pp.1670-81.
Ott, I. and Gust, R. (2007) ‘Non Platinum Metal Complexes as Anti-cancer Drugs’, Archiv der Pharmazie, vol. 340, iss. 3, pp.117-126.
Birch, N.J. (1999) ‘Inorganic Pharmacology of Lithium’, Chemical Reviews, vol. 99, issue 9, pp. 2659-82.
Further information on leishmaniasis
Roychoudhury, J.and Ali, N., 2008 ‘Sodium Stibogluconate: Therapeutic use in the Managment of Leishmaniasis’ Indian Journal of Biochemistry and Biophysis, vol. pp.16-22.
Frézard, F., Demicheli, C. and Ribeiro, R.R., (2009) ‘Pentavalent antimonials: new perspectives for old drugs’, Molecules, vol. 17, no. 7, pp. 2317–36.
Further information on trypanosomiasis can be found at either of the following links:
Sanchez-Delgado R.A.; Anzellotti A., (2004), Metal Complexes as Chemotherapeutic Agents Against Tropical Diseases: Trypanosomiasis, Malaria and Leishmaniasis, Mini Reviews in Medicinal Chemistry, Vol. 4, No. 1, pp. 23-30.
Gold complexes as potential anti-parasitic agents by M. Navarro (Navarro, 2009). Although this focuses in particular on the use of gold it gives a good overview of the subject area and includes other examples.
B.S. Atiyeh, B.S., Costagliola, M., Hayek, S.N. and Dibo, S.A. (2007) ‘Effect of silver on burn wound infection control and healing’, Burns, vol. 33, iss. 2, pp. 139-148.
Edwards-Jones, V. (2009) ‘The benefits of silver in hygiene, personal care and healthcare’, Letters in Applied Microbiology, vol. 49, iss. 2, pp. 147-152.
Monteiro, D.R., Gorup, L.F., Takamiya, A.S., Ruvollo-Filho, A.C., de Camargo, E.R. and Barbosa, D.B. (2009) ‘The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver’, International Journal of Antimicrobial Agents, vol. 34, iss. 2, pp. 103-110.
Silver, S. (2003) ‘Bacterial silver resistance: molecular biology and uses and misuses of silver compounds’, FEMS Microbiology Reviews, vol. 27, iss. 2-3, pp. 341-353.
Silver, S., Phung, L.T. and Silver, G. (2006) ‘Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds’, Journal of Industrial Microbiology and Biotechnology, vol. 33, no. 7, pp. 627-634.
Shechter, Y., Goldwaser, I., Mironchik, M, Fridkin. M. and Gefel, D. (2003) ‘Historic perspective and recent developments on the insulin-like actions of vanadium: towards developing vanadium-based drugs for diabetes’, Coordination Chemistry Reviews, vol. 237, iss. 1-2, pp. 3-11.
Thompson, K.H, Lichter, J., LeBel, C., Scaife, M.C., McNeill, J.H. and Orvig, C. (2009) ‘Vanadium treatment of type 2 diabetes: A view to the future’, Journal of Inorganic Biochemistry, vol.103, iss. 4, pp. 554-558.
Malemud CJ., Front Biosci. 2006 May 1; 11:1696–701. Review.
Metal-Ceramic Alloys in Dentistry: A Review
Roberts, Howard W.; Berzins, David W.; Moore, B. Keith; Charlton, David G., Journal of Prosthodontics, Volume 18, Number 2, February 2009 , pp. 188–194(7)
Grateful acknowledgement is made to the following sources for permission to reproduce material in this text.
Figure 9.3a: James Stevenson/Science Photo Library; Figure 9.3b: Royal Berkshire NHS Foundation Trust; Figures 9.4, 9.5, 9.7: Richard Wellings/University Hospitals Coventry and Warwickshire NHS Trust; Figure 9.8: El Saghir, N.S. et al. (2005) ‘Trauma-associated growth of suspected dormant micrometastasis’, Biomedcentral.com. Springer Science and Business Media; Figure 9.10: Cohen, M.S. and Bookheimer, S. Y. (1994) ‘Localization of brain function using magnetic resonance imaging’, Trends in Neurosciences, vol. 17. Elsevier Science; Figure 9.11: Dr Krish Singh, Aston University; Figure 9.12: Kosuke, I. et al. (2001) ‘Asymmetry of parietal lobe activation during piano performance-a high field functional magnetic resonance imaging study’, Neuroscience Letters, vol. 309, Issue 1, 17 August 2001. © Elsevier Science Limited; Figures 9.14, 9.15, 9.16 and 9.17: Nigel Williams/University Hospitals Coventry and Warwickshire Trust; Figure 9.16: G E Healthcare; Figure 9.20: Morgan, G.F., Nowotnik, D.P. (1999)’Development of dopamine transporter imaging agents for the diagnosis of Parkinson’s disease’, Drug News & Perspectives, 1999, 12(3): 137. Prous Science; Figure 9.21a: D Marta S. Walters, University of California, Santa Barbara and Professor Spencer W. Brown, University of California Berkley; Figure 9.21b: Physics Part 2. 1960/1962. John Wiley & Sons Ltd.; Figure 9.21c and d: Professor Barnett Rosenberg; Figure 9.30: Suo, Z., Lippard, S.J. and Johnson, K.A. (1999) ‘Single d(GpG)/cis-Diammineplatinum(II) Adduct-Induced Inhibition of DNA polymerization’, Biochemistry, vol. 38, no. 2. The American Chemical Society; Figure 9.31: Mike McCormick; Figure 9.32: Czarnota, G.J. et al. (1990) ‘Ultrasound imaging of apotosis: high-resolution non-invasive monitoring of programmed cell death in vitro, in situ and in vivo’, British Journal of Cancer, 1990, 81(3). Cancer Research Campaign.
Every effort has been made to contact copyright holders. If any have been inadvertently overlooked the publishers will be pleased to make the necessary arrangements at the first opportunity.
Nurse
Okay, Alan. I’m going to take some pictures of your head now, okay? So what I’m going to do is I’m just going to raise the bed up and get you into position.
Liz Parvin
Alan is now being placed in the CT scanner and the radiographer uses a laser beam to place his head in precisely the right location.
Nurse
So what I want you to do there is keep nice and still.
Liz Parvin
CT scanning is essentially an X-ray procedure. But the X-ray source is rotated around the patient and the intensity recorded on the opposite side of the patient. Using data from a large number of angles a computer reconstruction can produce a two-dimensional map of the tissues in a slice of the body.
Nurse
So what we’re going to do now is choose the protocols that we are going to use to do the head so we are going to click on the appropriate parts of the body here so we click on head and then we are going to an adult brain. Okay. The scan’s just about to start if you can just keep nice and still there, okay?
Liz Parvin
Planing is done by taking a pilot scan. The patient is moved through the gantry, the source remains stationary on one side. This produces an image similar to a planar X-ray. This pilot scan is taken in what is called the sagittal plane.
Nurse
We are going to do thinner sections through the base of the skull and then we are going to do wider ones as we go through towards the head at the top of the skull there. So now we can do our actual scan okay and then we can produce the actual images that the radiologist will look at.
So we can scroll through the images that we’ve just got okay and we can alter the grey levels of the images so that we can see different bits of anatomy. What we will do is we will look at the actual brain windows first so we are going to go through okay we are just looking basically for any asymmetry, any abnormalities. When we get further up we can change the window levels so we can see the ventricles better. That’s going through there. Now we’re at the top. And as this patient has got suspected base of the skull fracture what we now do is go back and we are going to change it to bone windows. We have highlighted the bone as white and everything else is now dark so it’s not seen so clearly. So again we are just looking through to see if there is any dark lines in the skull which could represent fractures.
Liz Parvin
The concern over the potential for injury to the top of the spine has been dismissed. But there is a concern that there may be some damage to the brain. So it’s decided to do an MRI scan.
Liz Parvin
Thankfully for this patient a CT scan has already shown that the top of the spine hasn't been fractured; damage to the brain has yet to be ruled out. Magnetic resonance imaging, or MRI, with its excellent soft tissue discrimination is the best way to check for this.
Nurse
It does make a very loud drilling noise okay so what we are going to do is pop these earphones on your ears.
Liz Parvin
To obtain images the patient is placed in a large, static magnetic field produced by a superconducting magnet. This powerful field, typically one-and-a-half teslas in strength, causes the nuclei of the hydrogen atoms in the body to line up. But they can also be flipped into another direction by a radiofrequency pulse. The way these nuclei then relax back to their original position depends on the environment of the hydrogen atoms. In other words, on tissue type. When imaging small parts of the body, specialised coils are used to detect the radiofrequency signal given off by the relaxing nuclei. Here a head coil is being used for this purpose. Laser alignment is used so that the patient can be moved to the correct position in the scanner. Because the powerful magnetic fields can disrupt pacemakers and cause heating of metal implants every patient must be carefully checked before entering the scanner room.
Nurse
Okay. So we are just going to start the scan now. So what we do first is an initial pilot scan so that we can exactly locate the patient within the scanner.
Liz Parvin
With MRI it’s possible to choose any direction for the image slices. In this case the radiographer is taking an axial pilot scan to give a series of coronal images.
Nurse
What we’ve done now is we’ve planned to scan through that area of this person’s head and we are going to scan from front to back there. So okay your first scan is just about to start. Each scan will take about five minutes okay and then after that we can have a look through the images and we can see what we’ve got.
Liz Parvin
There are many different imaging sequences that can be used but most of them produce an image where the intensity depends mainly on one of three characteristics: T1, T2 or proton density. The intensity in these images depends largely on T2 - the spin-spin relaxation time. Substances with a long T2, such as water, appear bright. This is very obvious if you look at the eyes in this image.
Nurse
We’ve now gone on to sagittal images. This is a T1 so we’ve got bright fat and the fluid’s dark on these images. This is a way that we can differential contrast to see different pathologies or anatomy. And we also scan in the axial plane so that’s head to toe. And then again this is T2 with bright fluid. You can see the eyes – and then finally these ones are proton density okay so this is weighting where each tissue is the protons that are actually there rather than any specific weighting.
Liz Parvin
Thankfully for this patient the images show no abnormality.
Liz Parvin
Thankfully for this patient a CT scan has already shown that the top of the spine hasn't been fractured; damage to the brain has yet to be ruled out. Magnetic resonance imaging, or MRI, with its excellent soft tissue discrimination is the best way to check for this.
Nurse
It does make a very loud drilling noise okay so what we are going to do is pop these earphones on your ears.
Liz Parvin
To obtain images the patient is placed in a large, static magnetic field produced by a superconducting magnet. This powerful field, typically one-and-a-half teslas in strength, causes the nuclei of the hydrogen atoms in the body to line up. But they can also be flipped into another direction by a radiofrequency pulse. The way these nuclei then relax back to their original position depends on the environment of the hydrogen atoms. In other words, on tissue type. When imaging small parts of the body, specialised coils are used to detect the radiofrequency signal given off by the relaxing nuclei. Here a head coil is being used for this purpose. Laser alignment is used so that the patient can be moved to the correct position in the scanner. Because the powerful magnetic fields can disrupt pacemakers and cause heating of metal implants every patient must be carefully checked before entering the scanner room.
Nurse
Okay. So we are just going to start the scan now. So what we do first is an initial pilot scan so that we can exactly locate the patient within the scanner.
Liz Parvin
With MRI it’s possible to choose any direction for the image slices. In this case the radiographer is taking an axial pilot scan to give a series of coronal images.
Nurse
What we’ve done now is we’ve planned to scan through that area of this person’s head and we are going to scan from front to back there. So okay your first scan is just about to start. Each scan will take about five minutes okay and then after that we can have a look through the images and we can see what we’ve got.
Liz Parvin
There are many different imaging sequences that can be used but most of them produce an image where the intensity depends mainly on one of three characteristics: T1, T2 or proton density. The intensity in these images depends largely on T2 - the spin-spin relaxation time. Substances with a long T2, such as water, appear bright. This is very obvious if you look at the eyes in this image.
Nurse
We’ve now gone on to sagittal images. This is a T1 so we’ve got bright fat and the fluid’s dark on these images. This is a way that we can differential contrast to see different pathologies or anatomy. And we also scan in the axial plane so that’s head to toe. And then again this is T2 with bright fluid. You can see the eyes – and then finally these ones are proton density okay so this is weighting where each tissue is the protons that are actually there rather than any specific weighting.
Liz Parvin
Thankfully for this patient the images show no abnormality.
John Duncan
The basic principle we’re making use of in using PET scanning is that, whenever a part of the brain works on some task, then blood flow increases in that brain area. And PET scanning is a way of measuring that increase in blood flow in different types of mental activity.
Doctor
This is the worst bit.
Matt Lambon-Ralph
PET stands for Positron Emission Tomography - it’s one of the best established techniques. A radioactive substance is injected into the blood stream.
Doctor
Okay that’s in position now so I'll just tape it down.
Matt Lambon-Ralph
It circulates all over the body including the brain. The head is carefully positioned into the centre of the PET camera.
Doctor
You’re in the right position for the scan now so I’ll bring the monitor into position.
Matt Lambon-Ralph
A coil inside the machine surrounds the head.
John Duncan
Okay, I'm just going to move the screen into position.
Matt Lambon-Ralph
It measures the intensity and source of radioactivity while the person performs a mental task.
John Duncan
So, as I explained, you’ll be giving your answers on this keyboard - use these two fingers of each hand - just rest them comfortably on the keys - and we’ll put the whole thing down on your stomach like that. All right.
Female
Thank you.
Doctor
Okay, I’m just going to flush this tube and connect you up so I can give you the radioactive water.
John Duncan
In our experiment we’re using an isotope of oxygen which has been attached to water so we inject radioactive water essentially into the bloodstream and then the PET camera is used to take a snapshot of where the radioactive concentration is highest in the brain, indicating high blood flow in that region and in that way to give us an idea of which part of the brain is responsible for the sort of activity that we’re asking the person to carry out during the scan.
Doctor
Okay are you ready, John?
John Duncan
Yeah.
Doctor
Cyclotron ready? Okay, after three. Three, two, one, go.
Matt Lambon-Ralph
The radioactive water is made on site; it takes two-and-a-half minutes to get it into the blood and to the brain. They have just forty-five seconds to measure brain activity while the radioactive isotope is at its most powerful. So the experiment has to be synchronised with this peak in radioactivity.
Doctor
Okay, the activity going there in about thirty seconds.
Matt Lambon-Ralph
One of the advantages of PET is that it measures activity anywhere in the brain but there’s a limit in the total amount of radiation each person can have. So a major disadvantage to PET scanning is that any one person can only be tested twelve times. Each scan gives a three-dimensional image of activity across the whole brain over forty-five seconds. This can be viewed as a series of two-dimensional slices.
John Duncan
On these slices the whiter it looks that means there’s high blood flow in that area and the blacker it looks it means that there’s low blood flow. So now this is a single snapshot - in order to get reliable results we’re going to average several of these together for each person and that immediately raises several problems. If a person has moved even slightly, just by a millimetre or less, that can seriously distort the analysis. So the first thing that you have to do is to align all of the different images from one person with one another so that they’re all totally exactly on top of one another and then we can average them together. So the next thing that we have to do is to get all the pictures from the different volunteers lined up with one another if you like. And the way that that works is to line each one up with a standard computerised standard brain if you like, so everything is rotated slightly and shifted slightly and expanded and contracted until they’re all totally lined up with one another. Then the results that you get look something like this.
Matt Lambon-Ralph
The combined images are compared, the problem-solving task gives one pattern of activity. The control task gives another pattern. Once the control activity is discounted, the remaining areas reflect activity specific to problem solving. Significant differences between the two patterns of activity are then shown as coloured areas on a computer model of the brain.
Matt Lambon-Ralph
PET is only one way to measure brain activity. The other well-known method is fMRI which stands for functional magnetic resonance imaging. This uses a technique that one might have if you’re a person going to hospital and you've bumped your head and they want a detailed picture of your brain - so an MRI machine. Typically they use a very powerful MRI machine and go after a specific signal that is a way of measuring brain activity. fMRI uses a strong magnetic field to measure levels of oxygen in the blood. When a brain region is active it takes up oxygen from the local blood supply. The resulting drop in oxygen levels provides an indirect measure of brain activity.
Doctor
Okay, I’ll just rest this on your tummy, hold that with your right hand.
Matt Lambon-Ralph
During a scan the person is placed in the MRI machine. The magnetic field is switched on and off rapidly to produce an image each time. This is a two-dimensional slice through the brain. A picture of the whole brain is built up from a series of these slices. Because many slices are taken in a short period of time, fMRI is able to measure not just where in the brain activity occurred, but also when this activity happened.
Adrian Owen
Essentially we can scan continuously if we like but typically we’d scan for ten or twelve minutes and then we can break that time period up to look at what was happening at individual stages. So instead of just looking at brain activation during a memory task in general, we can say, well what was happening during encoding or what was happening while the person was trying to remember the information? Or what was happening when they were trying to recall the information? So essentially we can cut up the task in terms of brain activation at different points. Because that tells us important things about cognitive function, because we can say, well what was the brain doing when the person was encoding the information, or what was the brain doing when the person was asked recall the information? As opposed to just saying, well what’s happening during a memory task in general? We can scan across a long period of time, so for example, one might look at learning, that’s something that’s previously been very difficult to do. Typically, if you’re learning a skill it might take you several minutes or even hours. There’s no reason why you couldn’t scan somebody even for a number of hours using fMRI and you could look at how the brain, how the brain activation changed during the course of learning.
Liz Parvin
The first step in radionuclide imaging is the preparation of the radiopharmaceutical. This must be carried out under stringent conditions of sterility. There are also strict guidelines to protect staff members from ionising radiation. The radionuclide used for most imaging techniques is Technetium-99m. This is extracted from a generator on a daily basis by passing saline solution through the generator - a process known as elution. Inside the generator an ion exchange process results in the production of a solution of sodium pertechnetate. Having drawn off the sodium pertechnetate, it is important to measure and record the activity of the eluate. In order to image a particular organ, technetium must be combined with a substance that targets that organ. To do this the sodium pertechnetate must be chemically reacted with an appropriate compound. Here the technetium needs to be combined with macro-aggregated albumen in order to target the lungs. To do this the appropriate quantity of pertechnetate is diluted with saline and then added to the pulmosis powder and shaken. This radiopharmaceutical will then be ready for injection into the patient after fifteen minutes.
Nurse
Could you tell me your name and address and date of birth please?
Liz Parvin
The patient’s identity must be checked by two members of staff before the injection is given. They must also check the radiopharmaceutical is the correct one for the procedure being carried out.
Nurse
What I’m going to do now is I’m actually going to give you an injection through here, okay? The injection I’m going to give you as I said is very slightly radioactive. It won’t make you feel strange. And you may feel this cold up in your arm.
Liz Parvin
A lead-covered syringe offers protection to the personnel performing the injection.The time between the injection of the radiopharmaceutical and imaging depends on the specific procedure. In some cases imaging is carried out immediately. In others there can be a delay of several hours.
Liz Parvin
This patient has been admitted to hospital with a suspected pulmonary embolism. To investigate this a VQ scan is going to be carried out. This examines both the blood supply, or perfusion, to the lungs and the ventilation of the lungs. Two radionuclides which emit gamma radiation at different energies are used for this procedure. The patient has already received an injection of technetium combined with macro-aggregated albumen or MAA. The particles of MAA tend to lodge in the very small capillaries of the lungs.
This generator contains rubidium-81, which decays to produce the gamma emitter krypton-81m. Air is passed through the generator and carries the krypton to the patient’s lungs. The half-life of krypton-81 is thirteen seconds so the gas expired poses little hazard to the staff.
Nurse
Okay. Keep nice and still. I’m going to start the picture now.
Liz Parvin
As the radionuclides decay the detected gamma rays gradually build up to form a useful image. The image on the left shows the technetium decay and therefore represents the perfusion in the lungs. The image on the right is formed by the gamma rays emitted by the krypton and shows ventilation.
Nurse
On the left-hand side of the screen we’ve got the perfusion, or the blood supply, image to the lungs. This side is the air supply or ventilation and the ventilation you can see the air mass there. It’s a posterior view, which means we are looking at it from the back. This is the left lung here and here and this is the right lung there and there. In order to get the two pictures at the same time we use a dual energy acquisition. This one’s the technetium energy which is a 140 keV and this is on is the krypton ventilation and that’s 190 keV. The camera is set up to recognise both the radionuclides at the same time.
Liz Parvin
A full VQ examination is completed by acquiring images from several different angles. It is possible to carry out gamma camera imaging by recording images from a large number of angles. These can be processed to produce tomographic images. This technique is known as SPECT.
Liz Parvin
This patient is being prepared to undergo a SPECT procedure to look at myocardial perfusion – that is blood supply to the heart muscle. It’s usual to scan the patient to look at the perfusion when the heart has been stressed and also when the heart is rested, and there can be several days between these two image acquisitions. Stressing of the heart is achieved in two ways: first by a pharmaceutical which has a similar effect on the heart to taking exercise, and also by squeezing a dumb bell. The heart needs to be monitored carefully during this period so an ECG is recorded.
Nurse
This is the bit that allows us to see to take some pictures to see what’s going on.
Liz Parvin
Once the heart has been stressed the radiopharmaceutical is injected. In this case the pharmaceutical being used is technetium-labelled tetrofosmin.
Nurse
That’s that part of the test finished now Mr Park. So I’m just going to disconnect you from all the machinery and I’m going to ask you wait in the waiting room because it takes this isotope up to an hour to reach your heart and then I’ll come and collect you and escort you through for your scan, okay?Hello Jeff – this is Mr Park. Mr Park this is Jeff and Jeff will be doing your scan so I’ll hand you over.
Nurse
If you’d like to lie on this bed on your back for me – I need you to put your arms just above your head for me now – just on either side of your ears – and that one as well.
Liz Parvin
In order to obtain tomographic images two gamma cameras must be rotated around the patient as close as possible to the heart. And the cameras are now trained to move around the patient as closely as possible without touching him.
The images are reconstructed from the data using the same back projection techniques that are used in CT scanning. They’re shown as slices through the heart in different directions with the white part of the image corresponding to high take-up of the radiopharmaceutical and the blue part representing low take-up.
Nurse
Right that’s it. We are all finished now. We can relax. We will be doing some pictures of your heart when it’s at rest and that will be later on. We will give you all the details of when we are going to do that in about ten minutes or so. First of all I’m going to check the data. Have a seat in the waiting room where you were before and I’ll come and let you know...
Nurse
Right. Here we have some processed images and we’re looking at the stressed and rest in the short axis – stress and rest in the horizontal long axis and stress and rest in the vertical long axis. And what we are now going to do is compare isotope distribution in the stress images and the rest images looking for reversible or irreversible defects.
Lesley Smart
Fundamental research has led to a most amazing anti-cancer drug discovery here at Michigan State University. Professor Barnett Rosenberg is a physicist interested in electric and magnetic fields. But his curiosity was aroused by a picture in a biology textbook.
Professor Rosenberg
Physicists rarely have any training or background in biology but this picture was a very fascinating one because it looked very much like to a physicist what you’d see if you put a bar magnet on a table and put a piece of paper on it and covered it with iron filings and you get that lovely pattern that we call a dipole field - dipole magnetic field or a dipole electric field – they both show the same patterns. Now the curious thing is that this was a picture of a cell in the process of dividing and it showed these lovely structures of the dipole field and I wondered whether there might not be a connection between the dipole field and the division process. To test this and it had been suggested by other people but it had never been tested so we decided to run a test on it.
Lesley Smart
The test equipment he assembled was a combination of electronics and a biological apparatus to grow cells in. Cells of this bacterium E. coli were being cultivated within an electric field. But instead of increased numbers of bacteria what they found surprised them. The bacteria had instead formed into long strands. What was causing this?
Professor Rosenberg
We concluded finally that it had nothing to do with the bacteria but it was something that the electric field, which was brought in with platinum electrodes into the chamber where the bacteria were growing, was somehow reacting to produce a chemical that went into the solution and it was this chemical that was causing these enormously long filaments, which is what we saw under the microscope when we looked at the bacteria coming out of the growth chamber, and it stopped cell division. Well – stopping cell division is precisely what you want to do if you can control the type of cell if you want to fight cancer because cancer is run away cell division. And so we had initially the idea that this might give us some ability to handle cells or control the growth of cells by the electric field effect but now what we found was instead of a physical effect a chemical effect.
Lesley Smart
If it was a chemical effect, the culprit had to be within the growth chamber. It was narrowed down to a molecule made by the chemical reaction between the nutrient - ammonium chloride – and the electrodes, which were made of platinum.
Professor Rosenberg
Platinum could be one of the possibilities, although we all believed that platinum was inert in a biological environment. Then when we had the chemicals isolated we then tried to find out which particular one of the platinum / ammonium chloride chemicals it was. And we tried finally to isolate it and it turned out to be a neutral molecule and it turned out to have a very specific structure.
Lesley Smart
This is the formula of the chemical they had produced. The platinum had reacted with the ammonium chloride and bonded to two chlorides and two ammonia groups. This molecule could have one of two flat structures where the platinum is surrounded by four ligands. This shape is known as square planar. One form has cis-chloride ligands, which means that they are on the same side of the molecule at right-angles to each other. This is commonly known as cis-platin. The other form has the same chemical formula but now the chloride ligands are trans or opposite one another. It’s known as trans-platin. Only the cis form of this chemical was active against cell growth.
Professor Rosenberg
We knew we had a method then of controlling cell division. It was not a physical method and we were disappointed in that but we had a chemical method. And therefore we went back to our original concept. Could we then control the growth of cancer cells by using this?
Lesley Smart
Next experiment was to inject doses of cis-platin into tumours in mice and compare results against a control group. The tumour decreased dramatically in size. The drug worked.
Professor Rosenberg
So this became then the focus of our interest because now we could treat a cancer in a mouse. The question was would it work in humans.
Lesley Smart
Professor Rosenberg and his colleagues wrote up the reports in the science journals and the medical research community went in to action on both sides of the Atlantic.
Lloyd Kelland
The drug then very rapidly, particularly for these days, went in to clinical trial in patients both in the United States and in Europe and the first hospital to look at cis-platin in Europe was in fact the Marsden Hospital next door to here where Doctor Eve Wiltshore very rapidly showed that the drug had activity in ovarian cancer in particular. And alongside that other trials showed that the drug was active in men with testicular cancer. Before the advent of cis-platin especially in the case of men with testicular cancer, the cure rate was something like one in ten. When cis-platin-containing regimes came into clinical practice the cure rate went up to something like nine in ten. So it had a dramatic effect and it was a very exciting time. It was probably the most active new cancer drug found in that period.
Lesley Smart
No drug is perfect. Cis-platin had side effects. Apart from nausea it could also cause kidney damage. It didn’t work against all cancers and most of all there was a huge gap in understanding. How did it work, and why didn’t the trans form of the same chemical have the same effect? Better understanding could possibly lead to better drugs and this interested top chemical research teams across the world, including a group at the Massachusetts Institute of Technology – MIT – led by Professor Stephen Lippard.
Professor Lippard
We were primarily a chemistry group beginning to learn something about macro molecules – proteins and nucleic acids. And we were particularly intrigued in the case of cis-platin with the fact that the cis-isomer, with the chlorides on the same side of the square plane, was active and the trans-isomer was inactive. And I’ve always been fascinated by problems in inorganic stereochemistry. And so it seemed that there would be a structure-function relationship that we ought to be able to sort out as chemists.
Lesley Smart
This is one of the tools the research chemist can use to discover details of the structure of molecules. It’s known as nuclear magnetic resonance spectroscopy, or NMR. The large shiny vessels are cryostats. They store the liquid helium, which cools superconducting magnets. These magnets generate huge magnetic fields and are so heavy that NMR facilities such this one at MIT are usually housed in the basement. Test samples are lowered into the magnetic field and radiofrequency energy is fired into them. Some atomic nuclei such as the 195 isotope of platinum possess a property known as spin. And the combination of the two fields can make such nuclei jump from one energy state to another. This in turn produces electronic signals unique to those particular atoms, giving information on their immediate environment in the molecule. NMR proved to be an immensely useful tool in helping to determine how cis-platin gets into the cells and what happened to it once it was inside. These chemical formulae show what the NMR results meant. Cis-platin hydrolyses step-wise to produce positively charged ions. First, one chloride ion is replaced by water to form a species with a single positive charge. And then the second chloride ion is replaced by another water molecule to form an ion with two positive charges.
Professor Lippard
And then when that happens the platinum becomes positively charged; the platinum complex becomes positively charged. And there would be a natural attraction then for it to bind and migrate to and bind to DNA which is a polyanion.
Lesley Smart
DNA is the complex molecule inside all living cells shown here in a very simplified form. Like all cells cancer cells grow by reproducing the DNA. And the assumption had to be that somehow cis-platin’s anti-cancer properties had to do with its interference in this process.
Professor Lippard
Every time the cell divides it has to reproduce its DNA, completely and accurately and in order for it to function it has to take the message that is encoded in the DNA, transcribe it into RNA, and ultimately translate that into proteins. We know that platinum blocks both of these processes, replication and transcription.
Lesley Smart
The vital portions of DNA are the centre groups. These are known as bases and they hold together the two strands of the double helix. The bases are adenine, thymine, cytosine and guanine. This is the chemical formula for guanine. What is important here is the nitrogen labelled as N7. It’s important because NMR was able to show that cis-platin could bond to the nitrogen at this site on the guanine base. This early work showed that most of the cis-platin formed what is called an adduct by linking to two neighbouring guanine bases on the same strand as the DNA through the N7 atom. We call this an intra-strand adduct, because the bonding is all on the same strand.
Lesley Smart
More unusually a very small amount of the cis-platin, less than five per cent, bonded to the DNA by straddling the two strands, making what is known as an inter-strand adduct. The trans-platin simply has the wrong geometry to form the linkages. Was this the beginning of the explanation?
A more precise technology such as X-ray crystallography was needed to sort things out but the great problem here is in growing good quality single crystals.
Professor Lippard
A major breakthrough in the laboratory with respect to getting X-ray crystallographic information came in the mid 1980s when a graduate student in my group – former graduate student in my group – Suzanne Sherman was successful in crystallising the smallest building block on DNA bound to cis-platin.
Lesley Smart
The crystalline DNA-platinum adduct was needed for this machine - the X-ray diffractometer. The wavelength of X-rays is comparable in size to the distance between planes of atoms in a crystal and the regular spacing inside the crystal diffracts the X-rays. Each plane of atoms in the crystal reflects the X-ray, producing a spot on a photographic plate. This, at first sight a rather unpromising, pattern of spots is the end result. Analysing both the position of the thousands of spots and their intensity produces these dramatic 3D pictures of the molecule itself where the position of each atom is known precisely. The first structure determined was a cis-platin bound to a single-stranded DNA. And it wasn’t until 1995 that Lippard’s group determined the crystal structure for the double-stranded DNA complex that we see here. Notice that cis-platin binds in the larger major groove. The platinum is still essentially square planar although the guanines are no longer parallel. What was this doing to the structure of the DNA molecule itself?
Professor Lippard
The DNA molecule underwent some rather interesting and profound structural changes. For one thing it underwent a rather significant bend ranging from fifty to eighty degrees off the linear direction that it ordinarily takes. For another, and probably this is the most significant, is that the minor groove of the DNA was substantially widened from the normal value in so called B or classical B formed DNA of about five to six angstroms up to ten or eleven angstroms. So opposite the platinum lesion was a widened, flattened minor groove producing a very different type of external structure than the one that one would find in DNA containing no chemical modification. And we believe that it is this altered structure of DNA that leads to the recognition of other factors in the cell which ultimately produce the anti-cancer activity.
Lloyd Kelland
It’s a very complicated area. Cis-platin binds to DNA and forms a variety of adducts on DNA. About ninety per cent of them, much the majority, are the intra-strand adducts on the same strand of DNA primarily against adjacent guanines on the DNA. But there are also inter-strand cross-links that cross the two strands of DNA which may be only two or so per cent but there are a group of people who believe that they are the more important in terms of biological significant whereas another group of people who believe that it’s the intra-strand adduct because of the higher numbers that think they are the more important.
Lesley Smart
Formation of the cis-platin-DNA adduct has damaged the DNA. If the DNA is not repaired then the cell cannot reproduce itself or replicate. Research has moved on to examine the cell mechanisms that deal with this damage.
Proteins are the key to processes such as the repair mechanism. Normally, damaged DNA is recognised and marked for repair which is done by cutting out the damage - so-called excision repair.
The bent platinated DNA seems to be recognised by other proteins which bind at the damaged site and prevent access by the repair proteins.
Professor Lippard
The class of proteins that bind to the cis-platin modified DNA, it turns out from gene sequencing and other experiments that we’ve done, generally contain what are called high mobility group or HMG domains.
Lesley Smart
The term high mobility group comes from this procedure known as electrophoresis. It’s used to identify and separate individual strands of DNA from a mix of all sorts. Cancer cells are cultured in the Petri dishes at body temperature – thirty-seven degrees Celsius. The cells are harvested and the protein extracted. They are then mixed with platinated DNA which has also been made radioactive by including a radioactive isotope of phosphorus - 32-phosphorus - in the phosphate groups.
The cultures are incubated for varying amounts of time and dyed a bright colour to make them visible.
The culture is dropped on to a gel and an electric field then applied. The DNA is negatively charged because of the phosphate groups and so it begins to travel down the gel towards the positively charged cathode. To make a permanent record of the experiment, a photographic film is put next to the gel. The radioactive phosphorus isotope blackens the film, thus indicating the positions of the DNA. As the repair mechanism operated during the incubation, bits of the DNA that had damaged by the cis-platin were excised. Because these pieces are smaller and lighter they travel faster and further down the gel. The longer the incubation ran, the more small excised bits of platinum-containing DNA can be seen as the DNA tries to repair itself. The technique can be used to study the effect of the HMG proteins on the repair mechanisms.
Professor Lippard
They could bind to the cis-platin modified DNA and shield or protect the adduct from excision repair. If this happened more efficiently in a tumour cell than in a normal cell of the same tissue that would be a wonderful mechanism for anti-cancer activity and we are working hard to evaluate that hypothesis.
Lesley Smart
This structure shows an HMG protein - the red tube – attached to a natural binding site for transcription. It binds to the minor groove but the similarity of the effect on the minor groove to the effect of cis-platin is striking. In both cases the minor groove becomes wider and shallower. Perhaps it is possible that the cis-platin DNA confuses the repair mechanism by this similarity and so escapes excision.
Lesley Smart
As well as understanding the mechanism the drive is also on to improve existing drugs.
Lloyd Kelland
It was realised very early on that the problem with cis-platin was one of chemical reactivity – the rate at which those chloride ligands leave the molecule and the whole idea behind carboplatin was to tone down that reactivity and in fact what is present in carboplatin instead of the chlorides is a cyclobutane dicarboxylato group which laboratory experiments have shown slows down the rate of equation, the rate at which those ligunds leave by about ten fold in carboplatin. And that has had a dramatic clinical effect in patients in that the kidney toxicity seen with cis-platin is almost totally absent with carboplatin and indeed the nausea and vomiting seen with cis-platin which is also a severe problem is much less with carboplatin as well.
Lesley Smart
Research now continues to make new platinum-containing drugs. One method is to employ so-called combinatorial methods. This is like one-pot cooking where all the ingredients are put into the pot together and then heated. In the set-up here it is possible to synthesise ninety-six new compounds at a time by simply adding different combinations of ligands to a platinum-containing starting material.
The reactions can take place under inert atmospheres such as argon or nitrogen or in the air. They can also take place at different temperatures in a range from –80 to 150 degrees Celsius. The reactants are mixed and after a suitable reaction time the products in each pot are filtered off, dried and then tested for anti-cancer activity.
Professor Lippard
We’ve been developing new methodologies that would allow us to test large families or libraries of platinum compounds, prepared in a combinatorial way, meaning that we would take mixtures of ligands including amines but extending it way beyond amines to examine thousands or maybe even millions of molecules for their ability to damage DNA and to produce altered structures which would lead to the binding of these HMG domain proteins and/or the specific blocking of gene function.
The cis-platin discovery is a huge success story where several sciences, physics, chemistry, biology and medicine have all made their contributions but the job is by no means over.
Lloyd Kelland
The big thing that we are still struggling to achieve is to broaden the activity of this class of drug into some of the other types of cancers. And in the next few years it’s a very exciting time because we are going to learn a lot more about how or whether this is achievable with the new molecules.
Professor Lippard
Ultimately the simple question is how does this work and when I give lectures I often entitle it cis-platin or platinum anti-cancer drugs – how might they work? I hope some day to be able to change the might to “do”. How do they work?
Professor Rosenberg
A large portion of science arose through serendipitous observations. People looking for something and finding something quite different than they anticipated. And the reason for this is we don’t know what the answer to the problem is that we are attacking. And therefore we come up with a guess and it’s very likely the guess is wrong. But something else might occur while we are studying whether the guess is correct or not tells us that no this other thing is the correct thing.
Lesley Smart
Metals are linked to health in another story of our own age and an all too common disease.
Dr Situnayake
Rheumatoid arthritis is the commonest inflammatory joint disease in the UK and world wide representing with a prevalence rate of about two per cent. Here we see the X-rays of the feet of a patient with rheumatoid arthritis and the key points to look at here - these are the bones of the feet here. These are the joints – the metatarsal phalangeal joints which, in layman’s terms, would be the balls of your feet. Essentially if you look at this joint here you will see that there is a joint space here which is filled with cartilage normally, preserved, but in all of these joints here you will see that the joint space is lost and what's happened here is that the cartilage within the joint has been lost as a result of the arthritic process and there are, there's thinning of the bone around the side of each of these joints here and complete loss of the cartilage within it so those are the advanced changes of rheumatoid arthritis.
This particular patient has an interesting history. She'd had rheumatoid arthritis for many years – more than twenty years – and had worn gold rings on that particular finger for many years. In fact before she had actually developed rheumatoid arthritis. And essentially-speaking she had one of the more severe forms of rheumatoid arthritis where virtually all of the joints, small joints in the hands, were badly damaged on the X-ray all except for one, which was the knuckle next to the ring. So we set about to design a research study comparing the joints, the small joints, of the hands in patients with rheumatoid arthritis who had either worn rings or who had not worn gold rings and essentially we seemed to show statistically that there was reduction in joint damage in the joints neighbouring the ring-wearing finger. So it appeared to be that the gold was somehow exerting a protective effect on the neighbouring joints, from damage.
Lesley Smart
Intriguingly, gold has been used for more than seventy years in conventional medicine to treat arthritis but in this form, as part of a large molecule - sodium aurothiomalate - injected intramuscularly. The detailed mechanism of how this drug acts is not yet understood but might this be linked to the effectiveness of the gold ring for some arthritis sufferers?
Dr Situnayake
We do have theories and they are simply that. One idea is that the actual gold itself, the molecule is absorbed through the skin, deposited in the local lymphatics and then drains into the neighbouring inflamed joint and thereby has its local anti-inflammatory effect. Of course, we do use gold in treatment of rheumatoid arthritis and there is a long-established practice for that. So that’s certainly a possibility. One other possibility which has been pointed out by people who looked at the results of our study when we published it was that it might be a local mechanical effect of the gold of the ring itself preventing the patient from using those joints as much as the other ones and thereby again protecting against damage. The bottom line is that we don’t yet know what the mechanism is.
Lesley Smart
The ongoing research into gold therapy may eventually give us the answers and allow new and better treatments for arthritis to be developed.